
Clinical Research
Taking part in research can help save lives
People rely on us to develop innovative medicines that have well-documented safety and effectiveness profiles. But behind every breakthrough are the patients and healthy volunteers who take part in our clinical trials. Trials are a vital part of this journey, helping us ensure our treatments meet the highest standards and are accessible to all.
What are clinical trials?
Clinical trials are research studies with volunteers designed to learn more about how our bodies respond to potential new medicines or treatments. These trials are important for advancing medicine and we’re grateful to the thousands of volunteers who participate in our clinical trials — making our research possible and shaping the future of healthcare.
At any one time, we conduct approximately

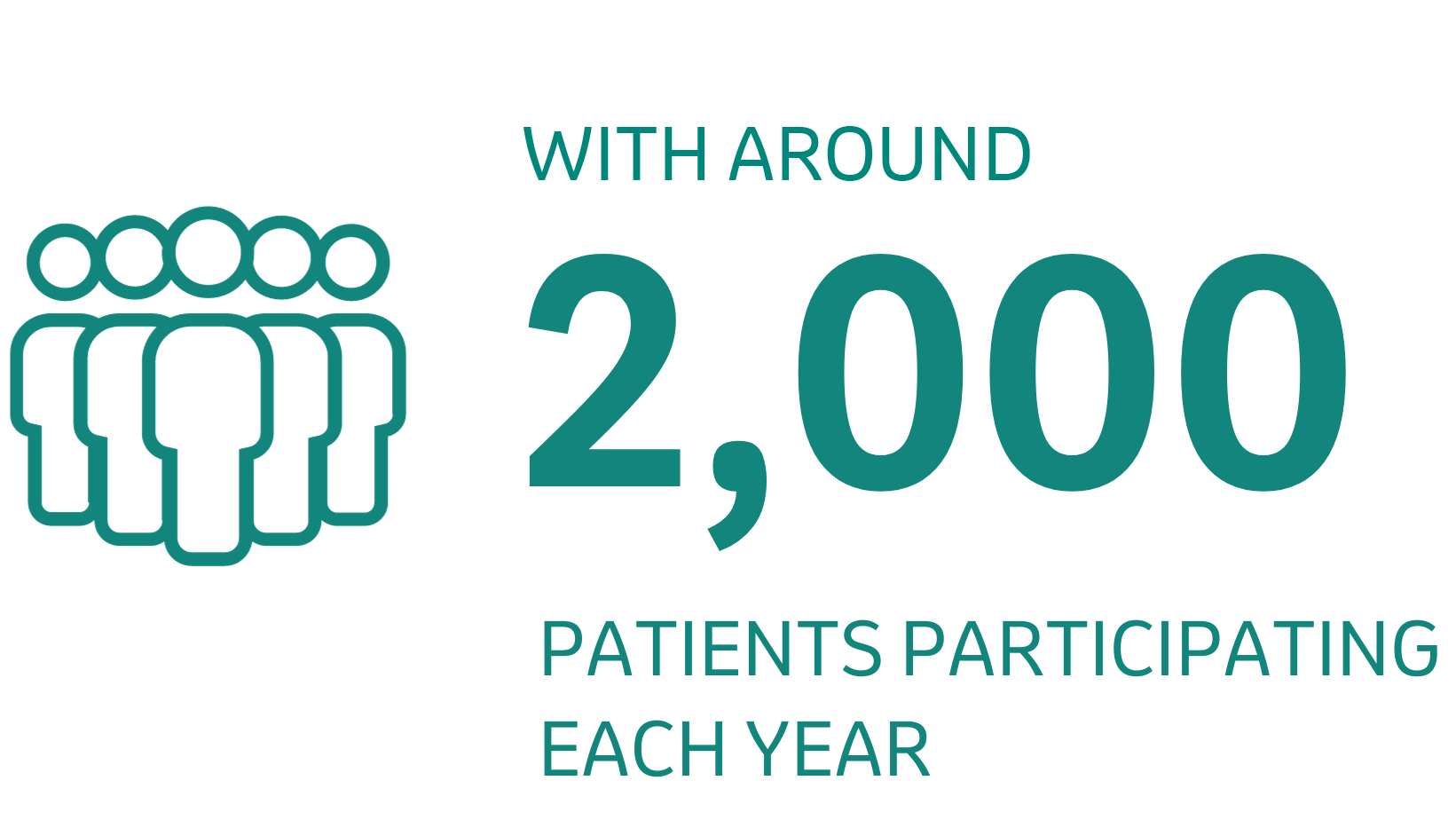
These volunteers play a vital role in the careful testing process medicines must undergo before receiving the necessary approvals. This process is designed to evaluate whether a new product should be approved for use in the broader population.
Find a trial

A list of clinical trials open in the UK is available through the National Institute for Health Research (NIHR)
Diversity and equity in our clinical trials
To develop lifesaving medicines for all, we have to start by doing research for all.
Diversity in clinical trials is an important part of our commitment to drive health equity. It is essential to developing treatments that address the needs of everyone. Different people may respond differently to the same treatment based on factors such as age, gender, ethnicity, and socioeconomic background. Ensuring diverse participation helps us understand these differences and create more inclusive healthcare solutions.

We are actively working to create equitable clinical trial research by:

Hear more from our team:
MSD’s commitment to increase diversity in our clinical trials

Michelle Szabo
Executive Director, UK Global Clinical Trial Operations
“At MSD UK, we are committed to making sure our research and medicines reflect the communities we serve. We’re grateful to our trial participants and site staff, whose help ensures our research represents a wide and diverse population.”

Rhianna Nelson-Forde
Clinical Research Associate
“Each day, we dedicate ourselves to understanding and meeting our patients’ needs. Behind every trial is a team committed to making participation more inclusive and representative, by supporting our patients every step of the way during their journey.”
Useful materials
View our leaflet on Understanding Clinical Trials
Visit our Patient Engagement page to learn about some of the other ways we are working with the patient

Our clinical trials are designed, conducted and monitored in adherence to the guidelines of The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).
MSD clinical trials adhere to the same standards whether they take place in the UK or elsewhere around the world.
CLINICAL TRIAL TRANSPARENCY
Regardless of their outcome, we register clinical trials at ClinicalTrials.gov. We have been posting results of clinical trials at the site since October 2008.
Code of Practice
The ABPI Code of Practice requires that companies in the UK register current and future trials within 21 days of enrolling the first patient, and results must be published within one year of marketing authorisation or one year from completion for marketed products. We are pleased to be meeting these requirements.
GB-NON-11729 | October 2025


