
COLLABORATIVE WORKING
MSD supports and partners with the NHS and healthcare organisations in a number of different ways.
Through a combination of Collaborative Working (multi-organisation) and Joint Working (direct to NHS provider partnering) we pool skills, experience and resources for the development and implementation of patient-centred projects. Our ability to continue with our long-standing collaborative efforts pivots on the Department of Health and Social Care's joint working mandate and enables us to discover and invent new ways, every day, to help patients, their families and their loved ones.
Select a project below to see more about how we partner or to find out more please contact us.
Read some of our Collaborative Working stories here.
MSD COLLABORATIONS
Oncology Collaborative Working
Breast & Gynae - Active Projects
Oxford TNBC Neo-adjuvant and Adjuvant Clinic Support
Project Title
Oxford TNBC Neo-adjuvant and Adjuvant Clinic Support
Organisations involved
Oxford University Hospitals NHS Foundation Trust (OUH) and MSD
Summary
To redesign the treatment clinics for patients within the triple negative breast cancer (TNBC) service at the OUH. This will enable the service to better meet the growing patient volumes and demand on resources. This will be achieved through the provision of £24,000 of funding by MSD to support the employment of a Pathway Coordinator. Further resources will be pooled as MSD will provide project management time, and the OUH will provide ANP project oversight for this service development initiative. The new Pathway Coordinator will take on the administerial duties that the ANP team and breast Oncologists currently undertake. Duties which require a disproportionate amount of valuable specialist nursing and oncologist time that could otherwise be spent on patient treatment and care. Employing a Pathway Coordinator will enable the ANP team to initiate and run clinics fully dedicated to the treatment and care of neo-adjuvant and adjuvant TNBC patients, as well as dedicated separate clinic time for patients on oral systemic anti-cancer therapy (SACT). Currently, these patients are all seen in the same clinic where there can be a lack of focus and specific holistic care aimed towards those patients undergoing neo-adjuvant and adjuvant treatment. The project duration will be for 12 months, over which time we expect to measure the capacity that is released in terms of ANP time freed up to focus on the running of the new ANP and Consultant led clinics.
Background
- The number of patients being referred for and diagnosed with cancer is increasing due to better detection, screening, and early diagnosis programmes. Patients are also living longer with cancer meaning that the patient volume is increasing. All of which is putting rising demand and pressure on cancer service capacity
- Cancer services are looking for ways to release capacity (measured as HCP time) from existing pathway processes so that they can do more activity with the same (or less) resource
- Treatment regimens are becoming increasingly more complex which puts additional pressures on the workforce within SACT Day Units, as more time is needed to prepare the regimens, and more time is needed for their administration to patients. These factors further compound the capacity challenge
- The volume of breast cancer patients is increasing in line with this trend and newer treatment regimens in the neo-adjuvant and adjuvant setting are leading to capacity challenges for cancer nurse specialists, ANPs and SACT delivery nurses as patient list sizes increase. This also leads to an associated increased admin burden that clinical staff get involved with to help expedite patients through the clinical pathway onto treatment
- The OUH treats around 420 patients with early breast cancer, including a significant number of patients with early TNBC annually. Across the South West and Thames Valley region, OUH has the poorest Cancer Waiting Times (CWT) performance and highest number of breaches across the 62 day and 31 day pathways
- Due to increase in patient volume and activity, the corresponding increase in administration and the duty of the nursing staff to complete this work, this leads to less patient facing time and reduced ability and opportunity to reduce waiting times
- There is a recognised workforce challenge within the NHS, which is reflected in the OUH where nurse recruitment challenges exist, meaning that the cancer services are frequently short staffed. Existing nursing staff are feeling the problems compunding as they have to perform ever increasing admin duties that could otherwise be done by a dedicated Pathway Coordinator
Project Objectives
- To support the employment of a Pathway Coordinator to undertake administerial duties currently being done by the Oncologists and Nurses.
- To measure the capacity of HCPs that is released with their time freed up to run dedicated neo-adjuvant / adjuvant clinics 1 day per week and dedicated, separate oral SACT clinics.
- To provide better patient experience as there will be dedicated clinic time to support the neo-adjuvant and adjuvant patients more holistically as they prepare for and embark on SACT.
- Improve service efficiency to ensure clinics are fully utilised, and patients are booked in for the appropriate clinics and within the appropriate timeframes.
- Create a business case to gain recurrent funding from the OUH for this Pathway Coordinator post to continue to support the running of the clinics and the breast cancer treatment pathway.
Benefits
Benefits to patients
- Improved experience of cancer treatment pathway
- Potentially start on treatment earlier with reduced waiting times
- Access to clinic appointments in a timely manner
Benefits to the OUH
- Clinical capacity of HCPs released that can be reallocated and dedicated to the running of the new clinics and provision of patient care
- Fully utilised clinics with patients booked in for the appropriate clinics and within the appropriate timeframes
- Project management support to develop a dedicated neo-adjuvant and adjuvant clinic as part of the TNBC service
- SACT treatment rates and cancer waiting times potentially changing as access is improved with patients potentially starting on treatment earlier
Benefits to MSD
- A better understanding of efficiency measures to improve neo-adjuvant and adjuvant TNBC clinics, and the cancer patient’s pathway
- Enhanced reputation of MSD through partnership work
- Potentially improved access to innovative treatments in line with NICE guidance which may, or may not include MSD medicines
Funding & Resources
This project is a shared contribution between Oxford University Hospitals NHS Foundation Trust (OUH) and MSD. The total project cost is £24,000
GB-NON-07972 | September 2023
Ovarian Cancer Maintenance Therapy clinic
Project Title
Ovarian Cancer Maintenance Therapy clinic
Organisations involved
MSD (UK) Ltd and The Christie NHS Foundation Trust
Summary
The project will be an eighteen-month pilot of a “one stop shop” dedicated Ovarian Cancer Maintenance Therapy (OCMT) Clinic at The Christie, with a proposed start date of February 2022. The service would be nurse led, supported by a pharmacist and with clinical expertise provided by an oncology consultant. The proposed pilot will provide a dedicated ovarian cancer maintenance therapy clinic which will concentrate clinical expertise in this specific group.
Background
The Christie manages patients from across Greater Manchester, with a population of 2.8 million (1), which is the largest cancer network in the UK. It also provides second opinions for patients nationally and additionally, there is a private patient service available through HCA Christie. The Christie treats ovarian cancer patients from across Greater Manchester and currently patients on ovarian cancer maintenance therapies are managed in the medic-led outpatient clinic together with patients on active chemotherapy regimens. The numbers of patients requiring this treatment are growing and there is a backlog of patients due to the Covid pandemic (1). The clinic will aim to manage these patients within a dedicated service, alleviating the backlog of patients.
Project Approach
The project aims to enable The Christie NHS Foundation Trust to successfully set up and implement a “one stop shop” dedicated ovarian cancer maintenance therapy clinic, and to share lessons with peers and the wider NHS. The service would be nurse led, supported by a pharmacist and with clinical expertise provided by the oncology consultant. There will be a mix of virtual and face to face appointments, and treatment to be offered at home where appropriate. Additionally, the clinic will aid COVID recovery plans, by alleviating the back-log of patients due to the additional nurse time provided within the 5 weekly sessions in the clinic.
Project Objectives
- The clinic will aim to treat 90% of eligible patients with ovarian cancer by the end of the pilot
- The Christie will capture and collate the data, and results will be reviewed, with success measured via patient experience
- Help improve patient experience through patients having a designated time for their appointment
- Aid COVID recovery plans through the additional nurse time provided by the clinic to support patient awaiting treatment
- Funding to be secured to continue the clinic post pilot
Benefits
Patients:
- Dedicated service and point of contact to manage their treatment
- Addressing patient concerns will continuously improve and develop the service for subsequent patients
- Patient experience data may prove sufficiently compelling to lead to wider adoption of the service in other Trusts and reduce variation across the NHS
- Dedicated service may improve treatment options, compliance and experience for patients
NHS
- Patients managed within a nurse led OCMT clinic may result in consultants having more time available to deal with complex cases
- Continuously improve the service (e.g. highlighting areas of need or dissatisfaction)
- Provide other hospitals and services evidence to form business cases to adopt a similar service
- Dedicated clinic to providing additional nurse time will aid with reducing post COVID backlog
MSD
- Lead consultant may be willing to share experience of working with MSD to support future partnerships with the wider NHS
- Reputational benefit from partnering with The Christie
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of the ovarian cancer maintenance therapy clinic project is that MSD may see more appropriate use of their NICE approved medicines
Funding
From MSD (or budget to be covered by MSD):
- MSD £45,150 (based on Band 8 nurse (c£60k salary) 0.5 WTE. 5 sessions per week (3 patient facing, 1 MDT, 1 admin)
- To be phased in over 2 years:
- 2022 £30,150
- 2023 £15,000
- Project management- £6,000 (1 day per month)
From the NHS:
- NHS Pharmacist – £45,000 (salary c£60k)
- Consultant expertise – £6,980 (salary c£98k) (0.5 days per week)
Total MSD £51,150
Total NHS £51,980
Total project= £103,130
GB-NON-05464 | November 2025
References:
- Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie NHS FT
Breast & Gynae - Completed Projects
University Hospitals of Birmingham (UHB) Breast Cancer Pathway Development Project (PDP)
Project Title
University Hospitals of Birmingham (UHB) Breast Cancer Pathway Development Project (PDP)
Organisations involved
University Hospitals of Birmingham (UHB)
Summary
There is an opportunity in Birmingham to optimise the breast cancer pathway to improve the service quality, service efficiency, productivity, and patient experience. The desired outcome of this project is an improved breast cancer pathway achievement of the breast cancer 28 day Faster Diagnostic Standard and the 31- and 62-day Cancer Waiting Time Targets. The project intends to run for approximately 6 months, The data set used for baseline and impact evaluation will the UHB Breast Milestone Dashboard created for the project.
Benefits Realised
Due to significant and unanticipated capacity issues within the University Hospitals Birmingham system which needed to take priority the outcome summary for the UHB breast cancer pathway PDP has not been initiated and has been delayed.
Funding & Resources
This was a project with shared time commitment from University Hospitals of Birmingham (UHB) & MSD
Lessons learnt
Creation of a formal contingency plan should any of the key stakeholders and MSD project manager have time capacity issues, leave their post or their organisation during the implementation of the project.
Publications
No publications are planned.
GB-NON-09618 | June 2024
Gastric - Completed Projects
Belfast Health and Social Care Trust Oesophageal Pathway Development Project (PDP)
Project Title
Belfast Health and Social Care Trust Oesophageal Pathway Development Project (PDP)
Organisations involved
MSD
Belfast Health and Social Care Trust
Summary
A national optimal oesophageal cancer pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all oesophageal cancer patients receive optimal cancer care. There was an opportunity in Belfast Health and Social Care Trust to optimise the oesophageal cancer pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project was an improved oesophageal cancer pathway and achievement of the oesophageal Cancer 31- and 62-day Cancer Waiting Time (CWT) targets. The project started in April 2022 and finished in June 2023.
Benefits Realised
Through implementation of MSD’s Pathway Development Programme with the Belfast Health and Social Care Trust team, MSD provided project management support to assess the current state of the oesophageal cancer pathway and provide a gap analysis comparing the pathway with the National Cancer Pathway. MSD then supported the implementation, led by the NHS, of improvement initiatives to close these gaps and assisted with the measurement of their impact. As a result of the project, the following benefits were realised:
Patient Benefits
- Optimisation of the oesophageal cancer pathway which allows patients to navigate through the pathway more efficiently potentially increasing the chance of a successful outcome if patients are diagnosed earlier on in the pathway. Refer to NHS benefits below to see number of days potentially saved.
NHS Benefits
- Pathology Pathway was reduced by 4-6 weeks vs current turnaround times which will impact 759 patients per year. This was done by:
- Belfast Health and Social Care Trust reviewing the outcomes of the PDP and procuring a new companion diagnostic testing equipment which will allow in house testing.
- All patients will be reflexed tested.
- Pathology – The project highlighted the need for training which was then implemented across Belfast Health and Social Care Trust.
- Overall treatment rates were not measured as part of this Pathway Development Programme
- % of patients achieving CWT:
- 31 day target improved from 89% June 2022 to 95% June 2023
- 62 day target improved from 0% June 2022 to 31% June 2023
MSD Benefits
- Better understanding of oesophageal cancer patient needs
- Enhanced reputation of MSD through partnership work. NHS stakeholders completed a survey following the PDP and the results are shown below:
- Average score of 8.5/10 and 8.5/10 was scored for being satisfied with MSD’s collaborative working project and believed MSD contributed to their organisation’s cancer service pathway respectively.
- Average score of 8/10 would recommend working in collaboration with MSD to others.
- Quotes taken from the survey when questioned, “what has improved in your service/pathway following this collaborative working project” from NHS Stakeholders:
- “Improved pathology processes”
- “Nothing specific to the part of the pathway I’m associated with but I can see other aspects are positively affected”
- When questioned on feedback of your experience of working in collaboration with MSD
- “Very useful to have additional capacity and an outside voice to support project”
- “MSD very positive about trying to improve cancer pathways and always approach things with a ‘solution’ orientated mindset”
- This collaboration may have enabled more patients to access innovative treatments in line with NICE guidance which may or may not have included MSD medicines
Funding & Resources
This was a project with shared time commitment from Belfast Health and Social Care Trust & MSD
Lessons learnt
- Oesophageal cancer and patient numbers during the project was defined as per the Northern Ireland Oesophago-Gastric Cancer Audit published 2021 measuring the quality of care for patients diagnosed 2018-2019.
- Communication is key between all parties which is especially important between departments to optimise decision making and establish the appropriate intervention for the patient as early as possible
- Regular touch points to ensure clarity of expectation and outcomes
- Clear plan of action and review within stakeholder meetings
- Despite the best efforts of the NHS and MSD to optimise the oesophageal cancer patient pathway, attainment of the Cancer Waiting Time targets remains challenging. The CWT performance for oesophageal cancer has improved against a backdrop of decreasing performance for other tumour types suggestive of even greater challenges within the system.
Publications
There are currently no plans to publish the outcomes of this project by the hospital trust as per the date of this summary.
GB-NON-08707 | February 2024
Southern Health and Social Care Trust Pathway Development Project (PDP)
Project Title
Southern Health and Social Care Trust Pathway Development Project (PDP)
Organisations involved
MSD
Southern Health and Social Care Trust
Summary
A national optimal oesophageal cancer pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all oesophageal cancer patients receive optimal cancer care. There was an opportunity in Southern Health and Social Care Trust to optimise the oesophageal cancer pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project was an improved oesophageal cancer pathway and achievement of the oesophageal cancer 31 and 62 day Cancer Waiting Time (CWT) targets. The project started April 2022 and finished June 2023.
Benefits Realised
Through implementation of MSD’s Pathway Development Programme with the Southern Health and Social Care Trust team, MSD provided project management support to assess the current state of the oesophageal cancer pathway and provide a gap analysis comparing the pathway with the National Cancer Pathway. MSD then supported the implementation, led by the NHS, of improvement initiatives to close these gaps and assisted with the measurement of their impact. As a result of the project, the following benefits were realised:
Patient Benefits
- Optimisation of the oesophageal cancer pathway which allows patients to navigate through the pathway more efficiently potentially increasing the chance of a successful outcome if patients are diagnosed earlier on in the pathway. Refer to NHS benefits below to see number of days potentially saved.
NHS Benefits
- Pathology Pathway was reduced by 4-6 weeks vs current turnaround times which will impact 140 patients per year. This was done by:
- Belfast Health and Social Care Trust reviewing the outcomes of the PDP and procuring a new companion diagnostic testing equipment which will allow in house testing.
- All patients will be reflexed tested.
- Southern Health and social care trust will send samples to Belfast Health and Social Care trust
- Endoscopy (including Oesphagogastro duodenoscopy – OGD’s) Did Not Attend (DNA’s) rates improved from 39 patients (5.80%) in June 2022 to 20 patients (2.19%) in June 2023. Attendance rates for endoscopy (including OGD’s) improved from 82.56% in June 2022 to 93.52% in June 2023 by contacting patients prior to the procedure with a reminder and explanation of the procedure.
- Triage – improved triage from 7 days on average to 5 days on average per patients by adapting internal processes.
- Direct to Test rates improved from 89% in June 2022 to 95% in June 2023 even with additional demand on the service by adapting internal processes impacting 140 patients
- Overall treatment rates were not measured as part of this Pathway Development Programme
- % of patients achieving CWT:
- 31 day target= 100% June 2023 (maintained at same level as 2022)
- 62 day target improved from 25% June 2022 to 50% June 2023
MSD Benefits
- Better understanding of oesophageal cancer patient needs
- Enhanced reputation of MSD through partnership work. Four NHS stakeholders completed a survey following the PDP and the results are shown below:
- Average score of 8/10 and 6.5/10 was scored for being satisfied with MSD’s collaborative working project and believed MSD contributed to their organisation’s cancer service pathway respectively.
- Average score of 7.75/10 would recommend working in collaboration with MSD to others.
- Quotes taken from the survey when questioned, “what has improved in your service/pathway following this collaborative working project” from NHS Stakeholders:
- “Better communication and networking between key stakeholders”
- “Better understanding of all roles that each team member does and how this affects the service as a whole”
- When questioned on feedback of your experience of working in collaboration with MSD
- “Great team to work with, very engaging and encouraging. A sense that they were very much invested in patient benefit”
- “The partnership working with MSD was conducive in linking with all stakeholders, identifying issues across the whole patient pathway and supporting the development of the RAG plan to work on areas to address the issues. The regular stakeholder meetings helped to review, update and further develop the work plan”
- “Excellent interaction and constructive feedback”
- This collaboration may have enabled more patients to access innovative treatments in line with NICE guidance which may or may not have included MSD medicines
Funding & Resources
This was a project with shared time commitment from Southern Health and Social Care Trust & MSD
Lessons learnt
- Oesophageal cancer and patient numbers during the project was defined as per the Northern Ireland Oesophago-Gastric Cancer Audit published 2021 measuring the quality of care for patients diagnosed 2018-2019.
- Communication is key between all parties which is especially important between departments to optimise decision making and establish the appropriate intervention for the patient as early as possible.
- Regular touch points to ensure clarity of expectation and outcomes.
- Clear plan of action and review within stakeholder meetings.
- Despite the best efforts of the NHS and MSD to optimise the oesophageal cancer patient pathway, attainment of the Cancer Waiting Time targets remains challenging. The CWT performance for oesophageal cancer has remained steady against a backdrop of decreasing performance for other tumour types suggestive of even greater challenges within the system.
Publications
There are currently no plans to publish the outcomes of this project by the hospital trust as per the date of this summary.
GB-NON-08711 | February 2024
Northern Health and Social Care Trust Pathway Development Project (PDP)
Project Title
Northern Health and Social Care Trust Pathway Development Project (PDP)
Organisations involved
MSD
Northern Health and Social Care Trust
Summary
A national optimal oesophageal cancer pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all oesophageal cancer patients receive optimal cancer care. There was an opportunity in Northern Health and Social Care Trust to optimise the oesophageal cancer pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project was an improved oesophageal cancer pathway and achievement of the oesophageal cancer 31 and 62 day Cancer Waiting Time (CWT) targets. The project started in April 2022 and finished June 2023.
Benefits Realised
Through implementation of MSD’s Pathway Development Programme with the Northern Health and Social Care Trust team, MSD provided project management support to assess the current state of the oesophageal cancer pathway and provide a gap analysis comparing the pathway with the National Cancer Pathway. MSD then supported the implementation, led by the NHS, of improvement initiatives to close these gaps and assisted with the measurement of their impact. As a result of the project, the following benefits were realised:
Patient Benefits
- Optimisation of the oesophageal cancer pathway which allows patients to navigate through the pathway more efficiently potentially increasing the chance of a successful outcome if patients are diagnosed earlier in the pathway. Refer to NHS benefits below to see number of days potentially saved.
NHS Benefits
- Pathology Pathway was reduced by 4-6 weeks vs current turnaround times which will impact 188 patients per year. This was done by:
-
- Belfast Health and Social Care Trust reviewing the outcomes of the PDP and procuring a new companion diagnostic testing equipment which will allow in house testing.
- All patients will be reflexed tested
- Northern Health and social care trust will send samples to Belfast Health and Social Care Trust
- Endoscopy – Oesophago-gastro-duodenoscopy (OGD) direct to test levels were maintained at a 50% level even though additional demand on the service by adapting internal processes, impacting 188 patients per annum.
- OGD waiting times improved June 2022 vs June 2023 for suspect cancer, urgent and routine even with additional demand on the service by additional planning of the weekly lists.
Suspect Cancer 2.6 weeks June 2022 vs 2.3 weeks June 2023
Urgent 32.9 weeks June 2022 vs 12.6 weeks June 2023
Routine 87 weeks June 2022 vs 32.6 weeks June 2023
- Dietetics – Reducing the turnaround time of 84% patients receiving nutritional supplements from 3-5 days to 0-2 days by changing request from phone to email. This will impact 188 patients per annum
- Overall treatment rates were not measured as part of this Pathway Development Programme
- % of patients achieving CWT:
- 31 day target= 100% June 2023 ( maintained at same level as 2022)
- 62 day target improved from 0% June 2022 to 50% June 2023
MSD Benefits
- Better understanding of oesophageal cancer patient needs
- Enhanced reputation of MSD through partnership work. Nine NHS stakeholders completed a survey following the PDP and the results are shown below:
- Average score of 9.11/10 and 7.56/10 was scored for being satisfied with MSD’s collaborative working project and believed MSD contributed to their organisation’s cancer service pathway respectively.
- Average score of 8.44/10 would recommend working in collaboration with MSD to others.
- Quotes taken from the survey when questioned, “what has improved in your service/pathway following this collaborative working project” from NHS Stakeholders:
- “Improved Direct to test rates, OGD waits reduced. More understanding of pathway across all MDT. Dietetic delays reduced”
- “It has allowed time to focus specifically on the service and bring the team together to review current ways of working”
- “Improved process for engaging with GPs regarding supplements and improved early referral to the CNS”
- “It has allowed time to focus specifically on the service and bring the team together to review current ways of working”
- “Faster prescription of nutritional supplements for our UGI patients via GP Regional dietetic agreement on streamlining/developing literature for our patients- dysphagia score specific”
- When questioned on feedback of your experience of working in collaboration with MSD
- “Positive experience and allowed the MDT to review individually their part in the patient pathway and how this feeds into the overall patient pathway and anyway that this can be improved”
- “It was extremely useful to have oversight of pathway – not just locally but on a Regional level too”
- “excellent working with MSD and potential areas for improvement identified”
- This collaboration may have enabled more patients to access innovative treatments in line with NICE guidance which may or may not have included MSD medicines
Funding & Resources
This was a project with shared time commitment from Northern Health and Social Care Trust & MSD
Lessons learnt
- Oesophageal cancer and patient numbers during the project was defined as per the Northern Ireland Oesophago-Gastric Cancer Audit published 2021 measuring the quality of care for patients diagnosed 2018-2019.
- Communication is key between all parties which is especially important between departments to optimise decision making and establish the appropriate intervention for the patient as early as possible
- Regular touch points to ensure clarity of expectation and outcomes
- Clear plan of action and review within stakeholder meetings
- Despite the best efforts of the NHS and MSD to optimise the oesophageal cancer patient pathway, attainment of the Cancer Waiting Time targets remains challenging. The CWT performance for oesophageal cancer has improved against a backdrop of decreasing performance for other tumour types suggestive of even greater challenges within the system.
Publications
There are currently no plans to publish the outcomes of this project by the hospital trust as per the date of this summary.
GB-NON-08708 | February 2024
Head and Neck - Active Projects
CP/GP Head and Neck Cancer Pathway Project
Project Title
CP/GP Head and Neck Cancer Pathway Project
Organisations involved
NHS Tayside
Summary
NHS Tayside, through the North Cancer Alliance, are working in collaboration with MSD to develop a communication pathway between community pharmacies in the Dundee city area and their local primary care practices for patients with suspected head and neck cancers (HNC).
The project will evaluate the effectiveness of a communication pathway between community pharmacy and primary care to determine whether it help to facilitate earlier patient review.
Background
The project is required because as there has been an increase in the incidence of HNC in Scotland over the last three decades making it the sixth most common cancer in Scotland and survival rates have shown little improvement since the 1980’s. The incidence of HNC is more prevalent in more deprived areas where there are sometimes issues accessing primary care however there is often a community pharmacy nearby which may be able to help identify any HNC symptoms and refer suspected cases directly to their local GP practice for follow up. 1,2,3,4
Project Approach
The project will be aiming to –
- Demonstrate that community pharmacy staff can accurately and reproducibly utilise communication pathways to encourage patient presentation to primary care for further assessment
- Track patients through their journey from point of contact with community pharmacy, where symptoms are first recognised, to GP consultation and any onwards referrals which will demonstrate the utility of a community pharmacy pathway
- Align with Scottish Government’s Recovery and Redesign Cancer action plan to potentially create a scalable model that can be reproduced across Scotland and potentially adopted for other tumour types
- Expand upon and help realise the full potential of the Pharmacy First Scotland policy and contribute to the Scottish Government’s vision for smoother patient pathways
Project Objectives
The project would aim to deliver –
- A communication pathway between around 20 community pharmacies and primary care services in the Dundee city area aimed at identifying HNC’s
- A written evaluation of the feasibility of engaging community pharmacies to identify potential cases of HNC’s and the effectiveness of the communication pathway
- A road map for other Health Boards and/or Scottish Government to emulate/scale the project
Benefits
Benefits/ Impact to patients
- Potential improved health outcomes
- Increased potential to receive treatment
- Reduced health burden for NHS Tayside citizens
- Reduction in health inequalities
Benefits to the NHS partner
- Potentially scalable Communication pathway that could be adopted by other Healthboards/Scottish Government
- Potentially reduced health costs in medications and follow up appointments
- Improved patient experience of a service run by NHS Tayside
- Expansion of existing skill base in Community Pharmacy
Benefits to MSD
- Potential increase in the number of patients eligible for therapeutic intervention by identifying HNC patients earlier
- Opportunity for MSD to help support the development of evidence to establish the benefit of utilising community pharmacy to identify HNC patients
- Opportunity to engage as a partner with NHS Tayside rather than just being seen as supplier of medicines to the healthcare system
Funding
This project involves a pooling of skills and resources between the NHS Tayside and MSD UK over 12 months.
MSD Contribution = £45,322; NHS Tayside Contribution = £37,622; Total Project = £82,944
November 2022
1. https://publichealthscotland.scot/publications/cancer-incidence-in-scotland/cancer-incidence-in-scotland-cancer-incidence-andprevalence-
in-scotland-to-december-2019/cancer-incidence-dashboard/ (Assessed September 2022)
2. http://eprints.gla.ac.uk/177913/ (Assessed September 2022)
3. Todd A, et al. BMJ Open 2014;4:e005764. doi:10.1136/bmjopen-2014-005764 (Assessed September 2022)
4. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/head-and-neck-cancers (Assessed September 2022)
Job Code GB-NON-06659 – Date of preparation November 2022
Head and Neck - Completed Projects
Swansea Bay University Health Board Head and Neck Pathway Development Project (PDP)
Project Title
Swansea Bay University Health Board Head and Neck Pathway Development Project (PDP)
Organisations involved
MSD-UK
Swansea Bay University Health Board
Summary
The aims of this project were to optimise the head and neck cancer pathway across Swansea Bay University Health Board (SBUHB) through service redesign with the head and neck cancer multidisciplinary team and focussing on the pathway from suspicion of recurrence through to subsequent treatment. MSD helped to project manage the implementation of this initiative through MSD’s Pathway Development Programme. The project started in May 2022 and finished in March 2023.
Benefits Realised
Through implementation of MSD’s Pathway Development Programme with the SBUHB team, MSD provided project management support to assess the current state of the recurrent head and neck cancer pathway. MSD then supported the implementation of improvement initiatives to close these gaps and assisted with the measurement of their impact. As a result of the project, the following benefits were realised:
Patient Benefits
- Optimisation of the recurrent head and neck cancer pathway which allows patients to navigate through the pathway more efficiently potentially increasing the chance of a successful outcome. Refer to NHS benefits below to see number of days potentially saved
- Patient experience was not measured as part of this project.
NHS Benefits
- Referrals and tracking:
- A ‘one-for-all’ request form given to MDT lead by pathology to standardise the request for PDL-1 testing
- PDL-1 test request form being sent directly to the referrals team rather than coming through pathologist initially. This standardisation will help to improve efficiencies and potentially minimise patient delays
- PDL-1 test requested at the point of MDT after imaging review rather than waiting until later in the pathway (potentially saving a week in the pathway)
- PDL-1 test sent back to SBUHB via email and to Oncology at the same time as pathology to ensure treatment decisions could be made as quickly as possible. This can reduce the pathway by up to 2 days
- Pathology:
- Blocks identified and marked at initial pathology diagnostics to show which block would be best for preparing of slides for PDL-1 testing. Prior to this the blocks were taken out of archive and viewed by the pathologist at a separate stage of the pathway to identify the blocks
- Due to the actions listed above the patient pathway has been shortened by approximately 3 weeks
- The proportion of patients that are experiencing this pathway shortening is increasing and is now realised in more than 50% of cases
- Overall treatment rates were not measured as part of this Pathway Development Programme
- Data is still being gathered to see the impact on the 62-day Cancer Waiting Times
MSD Benefits
- Better understanding of head and neck cancer patient needs
- Enhanced reputation of MSD through partnership work. Two NHS stakeholders completed a survey following the PDP and the results are shown below:
- Average score of 8.5/10 and 8/10 was scored for being satisfied with MSD’s collaborative working project and believed MSD contributed to their organisation’s cancer service pathway respectively
- Average score of 8/10 in the likelihood of working with MSD and the pharmaceutical industry in the future (up from 5.5/10 prior to the PDP)
- Quotes taken from the survey when questioned, “what has improved in your service/pathway following this collaborative working project” from NHS Stakeholders:
- “Know where the delays are in the work flow”
- “Highlighted awareness of importance of smooth PDL1 pathway and highlighted areas of immediate improvement (generic email to submit requests already active) and to request at earlier points in the pathway”
- When questioned on feedback of your experience of working in collaboration with MSD:
- “MSD personnel were professional and provided helpful feedback”
- “Professional, thorough, personable and practical”
- This collaboration may have enabled more patients to access innovative treatments in line with NICE guidance which may or may not have included MSD medicines.
Funding & Resources
This was a project with shared time commitment from Swansea University Health Board & MSD-UK
Lessons learnt
- Communication is key between all parties which is especially important between departments to optimise decision making and establish the appropriate intervention for the patient as early as possible
- Regular touch points to ensure clarity of expectation and outcomes
- Clear plan of action and review within stakeholder meetings
- Waiting times on the recurrent head and neck pathway remain challenging. There are significant workforce challenges across Swansea Bay University Health board
Publications
There are currently no plans to publish the outcomes of this project by the health board as per the date of this summary.
GB-NON-08642 | December 2023
Aneurin Bevan University Health Board Head and Neck Pathway Development Programme (PDP)
Project Title
Aneurin Bevan University Health Board Head and Neck Pathway Development Programme (PDP)
Organisations involved
Aneurin Bevan University Health Board
MSD-UK
Summary
The aims of this project were to optimise the head and neck cancer pathway across Aneurin Bevan University Health Board (ABUHB) through service redesign with the head and neck cancer multidisciplinary team and focussing on the pathway from suspicion of recurrence through to subsequent treatment. MSD helped to project manage the implementation of this initiative through MSD’s Pathway Development Programme. The project started in February 2022 and finished in February 2023.
Benefits Realised
Through implementation of MSD’s Pathway Development Programme with the ABUHB team, MSD provided project management support to assess the current state of the recurrent head and neck cancer pathway. MSD then supported the implementation of improvement initiatives to close these gaps and assisted with the measurement of their impact. As a result of the project, the following benefits were realised:
Patient Benefits
- Optimisation of the recurrent head and neck cancer pathway which allows patients to navigate through the pathway more efficiently potentially increasing the chance of a successful outcome. Refer to NHS benefits below to see number of days potentially saved
- Patient experience was not measured as part of this project.
NHS Benefits
- Referrals and tracking:
- A ‘one-for-all’ request form given to MDT lead by pathology to standardise the request for PDL-1 testing
- PDL-1 test request form being sent directly to the referrals team rather than coming through pathologist initially. This standardisation will help to improve efficiencies and potentially minimise patient delays
- PDL-1 test requested at the point of MDT after imaging review potentially reducing the pathway by up to 7 days
- PDL-1 test sent back to ABUHB via email and to Oncology at the same time as pathology to ensure treatment decisions could be made as quickly as possible. This can reduce the pathway by up to 2 days
- Pathology:
- The health board is continuing to investigate the move to digital pathology to assist and alleviate issues with having multiple sites across pathology services. This project further highlighted the business case and initial work that had already been completed
- Blocks identified and marked at initial pathology diagnostics to show which block would be best for preparing of slides for PDL-1 testing. Prior to this the blocks were taken out of archive and viewed by the pathologist at a separate stage of the pathway to identify the blocks. This will reduce the pathway by 2 days
- Blocks now sent to an alternative reference centre for PDL-1 testing rather than ABUHB having to prepare slides. This has alleviated preparation capacity for ABUHB and the average turnaround time for PDL-1 tests being received has reduced from 40 days (Apr 2022) to 12.83 days (June 2023)
- Overall treatment rates were not measured as part of this Pathway Development Programme
MSD Benefits
- Better understanding of head and neck cancer patient needs
- Enhanced reputation of MSD through partnership work. Four NHS stakeholders completed a survey following the PDP and the results are shown below:
- Average score of 8/10 and 7.5/10 was scored for being satisfied with MSD’s collaborative working project and believed MSD contributed to their organisation’s cancer service pathway respectively
- Average score of 9.67/10 in the likelihood of working with MSD and the pharmaceutical industry in the future (up from 3.67/10 prior to the PDP)
- Quotes taken from the survey when questioned, “what has improved in your service/pathway following this collaborative working project” from NHS Stakeholders:
- “Engagement of the pathology department has been the most helpful aspect. It’s too early to see whether changes have resulted in improvement yet”
- “Our understanding of the workflow from surgeons to laboratory”
- “I am not sure what has improved. we are struggling with all the cancer pathways so prioritising one in isolation has a negative impact on the others”
- When questioned on feedback of your experience of working in collaboration with MSD:
- “I was impressed with the feedback MSD made summarising the changes that would benefit the service and MSD enabled good communication between Cellular Pathology admin staff, Clinicians and pathologists”
- “I was very impressed with MSD”
- This collaboration may have enabled more patients to access innovative treatments in line with NICE guidance which may or may not have included MSD medicines.
Funding & Resources
This was a project with shared time commitment from Aneurin Bevan University Health Board & MSD-UK
Lessons learnt
- Communication is key between all parties which is especially important between departments to optimise decision making and establish the appropriate intervention for the patient as early as possible
- Regular touch points to ensure clarity of expectation and outcomes
- Clear plan of action and review within stakeholder meetings
- Waiting times on the recurrent head and neck pathway remain challenging. There are significant workforce challenges across Aneurin Bevan University Health board
Publications
There are currently no plans to publish the outcomes of this project by the health board as per the date of this summary.
GB-NON-07847 | October 2023
South Yorkshire and Bassetlaw Cancer Alliance Head & Neck Pathway Development Project (PDP)
Project Title
South Yorkshire and Bassetlaw Cancer Alliance Head & Neck Pathway Development Project (PDP)
Organisations involved
South Yorkshire and Bassetlaw Cancer Alliance: Sheffield Teaching NHS Foundation Trust, Barnsley Hospital NHS Foundation Trust, The Rotherham NHS Foundation Trust, Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust and Chesterfield Royal Hospital NHS Foundation Trust & MSD-UK
Summary
A national head and neck cancer best practice timed pathway (BPTP) has been created with the intention of improving patient experience through promoting equality in cancer care and ensuring all head and neck cancer patients receive optimal cancer care. There was an opportunity in South Yorkshire to optimise the head and neck cancer pathways in line with the BPTP to improve the service quality, service efficiency, productivity and patient experience. Also, to improve cross-functional working relationships across all South Yorkshire and Bassetlaw Cancer Alliance Trusts including but not limited to ENT (ear, nose and throat) and OMFS (oral maxillofacial surgery) departments. The desired outcome of this project was an improved efficiency of the regional diagnostic head and neck cancer pathway aligned with the BPTP milestones, 28 day Faster Diagnostic Standard (FDS) and the 31- and 62-day Cancer Waiting Time (CWT) Targets. The primary objective was to implement the changes in the 5 foundation hospital trusts within the South Yorkshire Cancer Alliance region in partnership with Chesterfield. The project began on 1st January 2022 and finished on the 31st December 2022.
Benefits realised
Through implementation of MSD’s Pathway Development Programme with the South Yorkshire and Bassetlaw Cancer Alliance team, MSD provided project management support to assess the current state of the head and neck pathway and provided a gap analysis contrasting the pathway with the BPTP. MSD then supported the implementation of improvement initiatives to close these gaps and assisted with the measurement of their impact. Also, MSD facilitated the formulation of service improvement meetings within each of the 5 diagnostic hospitals. As a result of the project, the following benefits were realised:
Patient Benefits
- Quicker diagnosis and time to treatment of head and neck cancer has been reported through this project potentially leading to improved patient outcomes, See NHS benefits below:
- Patient experience was not measured
NHS Benefits
- Barnsley Hospital NHS Foundation Trust
- Referral of patient to when first seen by oncologist reduced from 9 days to 5 days
- Rotherham NHS Foundation Trust CT
- Reporting has reduced from 6 days to 4 days
- Sheffield Teaching Hospital NHS foundation Trust
- ENT referral of patient to when first seen by oncologist reduced from 10 days to 6 days
- ENT patients are now reviewed at MDT on day 35 vs day 40
- OMFS patients are now reviewed at MDT on day 36 vs day 40
- Doncaster and Bassetlaw Teaching Hospitals NHS Foundation Trust and Chesterfield Royal Hospital NHS Foundation Trust
- No data was collected
- Achievement of CWT & FDS was analysed from the 62- and 28-day CWT target performance data concluded the following:
- The 62-day CWT in January 2022 was 47.1% vs 52.9% in December 2022
- The 28-day FDS in January 2022 was 74% vs 77% in December 2022
- Data was an amalgamation of all the trusts within the South Yorkshire and Bassetlaw Cancer Alliance
- Treatment rates were not measured as part of this project
MSD Benefits
- Better understanding of head and neck cancer patient needs
- Enhanced reputation of MSD through partnership work. Four NHS stakeholders completed a survey following the PDP and the results are shown below:
- Average score of 8.75/10 were satisfied with MSD’s collaborative working project
- Average score of 8/10 believed MSD supported their organisations development or improved cancer service
- Average score of 3.25/10 to unlikely work with MSD and the pharmaceutical industry prior to the collaborative project which then changed to an average score of 8.25/10 in favour of collaboration following the project.
- Average score of 8.5/10 would recommend working in collaboration with MSD to others
- Quotes from NHS Stakeholders taken from the survey when questioned ‘what has improved in your service/pathway following this collaborative working project’:
- “Improvement in achievement of best practice timed pathway milestones, significant improvements in engagement with service improvement methodology and increased staff morale within the CDG leadership team”
- “Improved understanding of the pathway and barriers, data collection to inform developments, collaborative working through working groups and progress towards meeting BPTP (best practice timed pathway) milestones”
- “The involvement of MSD has resulted in vastly improved cohesion, consistency and efficiency across a number of aspects of the head and neck pathway in South Yorkshire & Bassetlaw”
- When prompted for ‘any feedback of your experience of working in collaboration with MSD’:
- “Wasn’t aware of the MSD collaboration projects prior to this”
- “Became a valued and welcome addition bringing expertise and previous experience”
- “Friendly and supportive in identifying the challenges that are faced in our area of work”
- This collaboration may have enabled more patients to access innovative treatments in line with NICE guidance which may or may not have included MSD medicines.
Funding & Resources
This was a project with shared time commitment from South Yorkshire and Bassetlaw Cancer Alliance, the participating Trusts & MSD.
Lessons learnt
- Importance of having access to tumour pathway steps timings data, and the challenges in extracting pathway timing data from Hospital IT systems. Recommendation – get agreement on data extract (both how and what) in project scoping phase.
- Wider NHS workforce challenges impacted on project delivery. Recommendation – engage project sponsors to ensure project work is prioritised throughout the duration of the project.
Publications
There are currently no plans to publish the outcomes of this project by the hospital trust as per the date of this summary.
GB-NON-07849 | August 2023
Northern Cancer Alliance (NCA) Head & Neck Pathway Development Project (PDP)
Project Title
Northern Cancer Alliance (NCA) Head & Neck Pathway Development Project (PDP)
Organisations involved
MSD-UK
Northern Cancer Alliance:
North Cumbria Integrated Care NHS Trust
Newcastle upon Tyne NHS Foundation Trust
County Durham and Darlington NHS Foundation Trust
South Tyneside and Sunderland NHS Foundation Trust
South Tees NHS Foundation Trust
Summary
A national Best Practice Timed Pathway for head and neck Cancer Diagnostics Pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all head and neck cancer patients receive optimal cancer care. There was an opportunity in the Northeast and North Cumbria to optimise the head and neck cancer pathways in line with the national timed head and neck cancer diagnostic pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project was an improved head and neck cancer pathway aligned with achievement of the head and neck cancer 28 day Faster Diagnostic Standard and the 31- and 62-day Cancer Waiting Time Targets. The project began on 1st January 2022 and finished on 31st December 2022
Benefits Realised
Due to changes in personnel and other unavoidable circumstances, data for the project is currently being gathered and analysed. The project benefits will be updated once data has been reviewed.
Funding & Resources
This was a project with shared time commitment from Northern Cancer Alliance & MSD
Lessons learnt
External challenges such as strike days within the NHS impacted on the delivery timescales of the project so the recommendation would be to factor in contingency time
Publications
There are currently no plans to publish the outcomes of this project by the hospital trust as per the date of this summary.
Job Code: GB-NON-07754 | Date of Preparation: July 2023
Royal Liverpool University Hospital NHS Foundation Trust Histopathology Head & Neck Pathway Development Project (PDP)
Project Title
Royal Liverpool University Hospital NHS Foundation Trust Histopathology Head & Neck Pathway Development Project (PDP)
Organisations involved
MSD UK and Royal Liverpool University Hospital NHS Foundation Trust
Summary
The objective of this project was to optimise the histopathology pathway in line with the head and neck national best practice timed pathway with an aim to improve the service quality, efficiency, productivity, and patient outcomes. This would contribute towards the achievement of the 31- and 62-day cancer wait time targets. MSD helped to project manage the implementation of this initiative through MSD’s Pathway Development Programme. The project began on 27th September 2022 and finished on 28th March 2023.
Benefits Realised
Through implementation of MSD’s Pathway Development Programme with Royal Liverpool University Histopathology team and Clatterbridge/ Aintree surgical and oncology teams, MSD provided project management support to assess the turnaround times of their histopathology testing pathway from MDT test-request to result reporting. A gap analysis was carried out and 3 areas of improvement were identified:
- Standardise the test requesting process:
- Switch test requests from multiple routes to one repository to increase efficiencies
- All test requests to be sent to pathology by latest Friday to get results for the following Wednesday MDT
- Standardise the process for locating and validating biopsies for testing:
- Surgeon and oncologist to include site of original biopsy, block, and year on the MDT form for future reference
- New way of working within the MDT:
- MDT outcome form to include PD-L1 result section which is to be declared at every MDT
- Biopsies gathered onsite would need to be reported within 7 days; 14 days for offsite
Patient Benefits:
Reduction in turnaround times will positively impact time to treatment decision for patients.
NHS Benefits:
Histopathology reports are now included on the MDT outcome form, which is visible to all MDT members, so all information is present at the MDT to discuss treatment options.
Report is also accessible on the ICE (integrated clinical environment system) which is available anytime to increase efficiency.
A reduction of 2 days in turnaround time and an increase in the number of cases being available for presentation at next MDT (Optimised pathway 77% v Routine Pathway 53%).
Achievement of 31- and 62-day treatment cancer wait time target was not measured.
Earlier referral, diagnosis and treatment of head and neck patients was not measured or used as a baseline as it was not relevant to histopathology.
Increase in treatment rates for head and neck was not measured or used as a baseline as it was not relevant to histopathology testing pathway.
Graph 1: From the 30 onsite cases the turnaround time and cases presented at MDT were compared between those following the routine pathway (17) and optimised pathway (13)
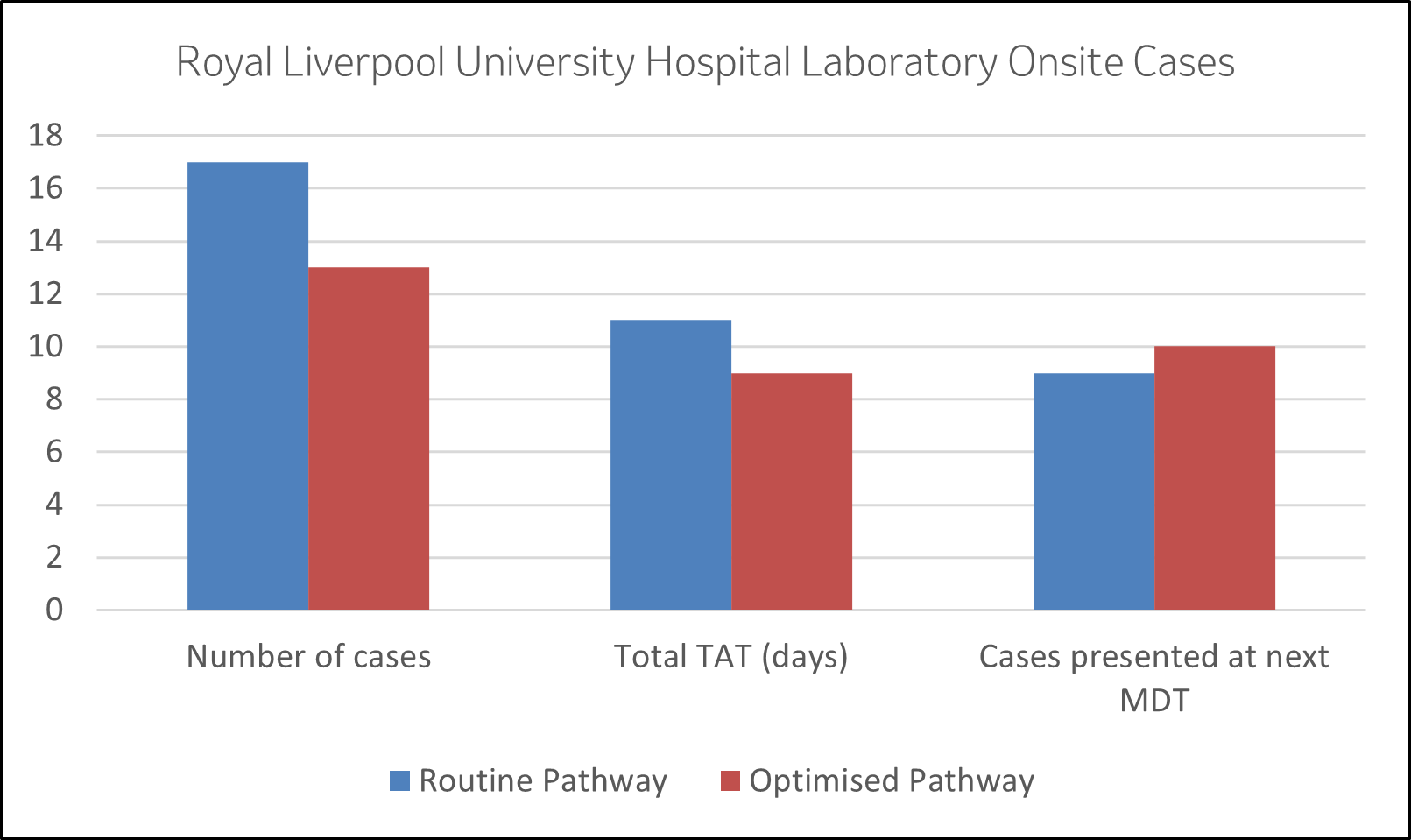
- New Average TAT is 9 days from 11 days
- More cases presented at next MDT (10 vs 9)
Total of 48 cases were evaluated, with 33 meeting reporting criteria and then 30 being managed onsite. These 30 cases were evaluated above.
MSD Benefits:
Better understanding of head and neck cancer patient and NHS needs.
Enhanced reputation of MSD through partnership work.
As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Quotes from NHS Stakeholders
“An enjoyable experience. The team provided excellent support, and this has improved our service”
“The PDL1 pathway has been streamlined. The system is more efficient”
“MSD colleagues have been professional and enthusiastic in this collaboration”
“Useful to meet service users in order to gain an understanding of their & our requirements”
Funding & Resources
This was a project with shared time commitment from both the NHS & MSD.
Lessons learnt
Tight project management and clear communication plan to ensure all stakeholders are aware of the initiative and the time demand needed for the project.
MSD workshops to be tagged onto meetings where you have a high attendance of healthcare professionals and stakeholders to increase attendance.
Following implementation of test requests to go through one repository, focus will now be on continually improving the process. It is currently managed by one healthcare professional; provisions need to be put in place in case of absence.
Publications
At the time of writing this summary there were no plans from the NHS organisation to publish this data.
Job Code: GB-NON-07560 | Date of preparation: June 2023
Dorset County Hospital NHS FT Head & Neck Pathway Development Project (PDP)
Collaborative Working Outcome Summary
This project was cancelled due to significant and unanticipated capacity issues within the Dorset system which needed to take priority
Project Title
Dorset County Hospital NHS FT Head & Neck Pathway Development Project (PDP)
Organisations involved
- Dorset County Hospital NHS FT
- MSD
Summary
The primary objective of this project was the optimisation of head & neck cancer pathways across Dorset. Specifically contributing towards: –
- An optimised head & neck cancer pathway aligned to the national optimal time head & neck pathway.
- Achievement of the head & neck cancer 28 day Faster Diagnostic Standard, 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets.
Benefits Realised
Due to significant and unanticipated capacity issues within the Dorset system which needed to take priority, the Trust made the decision to cancel the project’s planned Head and Neck Cancer Service pathway development workshops, the mainstay of the project plan
Funding & Resources
This was a project with a shared time commitment from University Hospitals Dorset NHS FT & MSD. No resources were committed from either side as project work had not commenced prior to the program’s cancellation.
Lessons learnt
- The importance of a strong sequential stakeholder engagement & communication plan to support time management
- The need to gain agreement from service management to clinical & administrative stakeholders for protected time to prioritise improvement project vs day to day clinical work during a pandemic
Publications
No publications had been planned
Job code: GB-NON-07310 | Date of prep: April 2023
University Hospitals Dorset NHS FT Head & Neck Pathway Development Project (PDP)
Collaborative Working Outcome Summary
This project was cancelled due to significant and unanticipated capacity issues within the Dorset system which needed to take priority
Project Title
University Hospitals Dorset NHS FT Head & Neck Pathway Development Project (PDP)
Organisations involved
- University Hospitals Dorset NHS FT
- MSD
Summary
The primary objective of this project was the optimisation of head & neck cancer pathways across Dorset. Specifically contributing towards: –
- An optimised head & neck cancer pathway aligned to the national optimal time head & neck pathway.
- Achievement of the head & neck cancer 28 day Faster Diagnostic Standard, 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets.
Benefits Realised
Due to significant and unanticipated capacity issues within the Dorset system which needed to take priority, the Trust made the decision to cancel the project’s planned Head and Neck Cancer Service pathway development workshops, the mainstay of the project plan
Funding & Resources
This was a project with a shared time commitment from University Hospitals Dorset NHS FT & MSD. No resources were committed from either side as project work had not commenced prior to the program’s cancellation.
Lessons learnt
- The importance of a strong sequential stakeholder engagement & communication plan to support time management
- The need to gain agreement from service management to clinical & administrative stakeholders for protected time to prioritise improvement project vs day to day clinical work during a pandemic
Publications
No publications had been planned
Job code: GB-NON-07265 | Date of prep: April 2023
Kidney - Active Projects
Streamlining Urology MDT meetings across the Southwest Urology Network of Hospitals
Project Title
Streamlining Urology MDT meetings across the Southwest Urology Network of Hospitals
Organisations involved
Peninsula Cancer Alliance and MSD
Summary
MSD intend to work in partnership with Peninsula Cancer Alliance (PCA) and the Southwest Urology network, to provide project management support to assess the performance of multi-disciplinary team meetings (MDTMs) across the region with a view to running improvement initiatives depending on the findings. By seeking endorsement and advocacy from the NHS Southwest Urology network, we will use MDT streamlining methods and principles to optimise these meetings and we will measure the impact of doing so.
Within the 12 months of planned project duration, our aims are to:
- Provide an analysis of the current functioning of each of the MDTMs across the region for Renal, Prostate and Bladder, including:
- Number of patients reviewed at each meeting and average time to review each patient
- At what frequency do meetings over-run
- Does each Trust run one meeting for all 3 tumour types or are the meetings separate
- Are Standards of Care used to standardise and expedite clinical decision making
- Average attendance by role
- Current challenges experienced in the running of the MDTMs
- Work with Urology leads and MDT coordinators from each Trust to define initiatives to streamline their MDTMs
- Assist with the implementation of these initiatives
- Measure and evaluate the impact of improvement and streamlining initiatives undertaken
- Demonstrate efficiency savings via improvement of meeting effectiveness
Background
Care by a MDT has long been the gold standard for patients with cancer, and a central part of the cancer pathway. However, much has changed in the cancer landscape over the last 20 years, as more sophisticated and personalised treatments are provided to a higher volume of patients, with increasingly complex cases1. Studies have found that there is generally not enough time in MDTMs to discuss more complex patients, with around half of patients discussed for two minutes or less2. An Independent Cancer Taskforce Report recommends that NHS England should encourage providers to focus specialist time in the MDTM on those cases which do not follow well-established clinical pathways3. This project serves to help address these recommendations.
Project Objectives
- Assessment of each MDTM across the region for Renal, Prostate and Bladder
- Analysis of current challenges and operating metrics of each MDTM
- Collaboration with each local team lead(s) to identify improvement initiatives
- Implementation of improvement initiatives to enable streamlining
- Evaluation of improvement initiatives and embedding of continuous improvement culture
Benefits
Benefits to the patient
- As a result of streamlined MDTMs, the patient may experience a faster cancer pathway
- Improved care processes and optimal treatment decisions made for each patient
Benefits to Peninsula Cancer Alliance
- Potential to introduce Standards of Care to streamline patient volume
- Capacity of Healthcare Professionals released through adoption of streamlining principles
- Help work towards meeting the quality actions stated in the Getting It Right First Time (GIRFT) Urology guidance
Benefits to MSD
- Enhanced reputation of MSD through partnership work
- A better understanding of the MDTM dynamics and NHS continuous improvement
Funding & Resources
This project is a shared contribution of time between Peninsula Cancer Alliance and MSD
- Streamlining Multi-Disciplinary Team Meetings Guidance for Cancer Alliances
- Cancer Research UK, “Meeting Patients’ Needs, improving the effectiveness of multidisciplinary team meetings”, January 2017
- Independent Cancer Taskforce Report, “Achieving World-Class Cancer Outcomes, a Strategy for England 2015-2020”, July 2015.
GB-NON-08626 | February 2024
South Eastern Health and Social Care Trust Renal Cell Carcinoma Pathway Development Project (PDP)
Project Title
South Eastern Health and Social Care Trust Renal Cell Carcinoma Pathway Development Project (PDP)
Organisations involved
MSD
South Eastern Health and Social Care Trust
Summary
An optimal Renal Cell Carcinoma pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all Renal Cell Carcinoma patients receive optimal cancer care. There is an opportunity in South Eastern Health and Social Care Trust to optimise the Renal Cell Carcinoma pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved Renal Cell Carcinoma pathway and achievement of the 31 and 62 day Cancer Waiting Time Targets. The project intends to run for approximately 12 months
Project Objectives
The primary objective of this project is the optimisation of Renal Cell Carcinoma pathways across South Eastern Health and Social Care Trust. Specifically contributing towards; –
- Achievement of the Renal Cell Carcinoma 31-day treatment target and 62 day referral to treatment Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the Renal Cell Carcinoma pathway in South Eastern Health and Social Care Trust
- Quicker diagnosis and treatment of Renal Cell Carcinoma and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway in Renal Cell Carcinoma across South Eastern Health and Social Care hospital sites resulting in
- Achievement of the Renal Cell Carcinoma 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of patients
- Increase in treatment rates for Renal Cell Carcinoma
- Optimisation of service delivery
MSD Benefits
- Better understanding of Renal Cell Carcinoma patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD
Job Code GB-NON-07411 | Date of Preparation – May 2023
Western Health and Social Care Trust Pathway Development Project (PDP)
Project Title
Western Health and Social Care Trust Pathway Development Project (PDP)
Organisations involved
MSD
Western Health and Social Care Trust
Summary
A Renal Cell Carcinoma pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all renal cell carcinoma patients receive optimal cancer care. There is an opportunity in Western Health and Social Care Trust to optimise the renal cell carcinoma pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved renal cell carcinoma pathway and achievement of the 31 and 62 day Cancer Waiting Time Targets. The project intends to run for approximately 11 months
Project Objectives
The primary objective of this project is the optimisation of renal cell carcinoma pathways across Western Health and Social Care Trust. Specifically contributing towards; –
- An optimised renal cell carcinoma pathway
- Achievement of the 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the renal cell carcinoma pathway in Western Health and Social Care Trust.
- Quicker diagnosis and treatment of renal cell carcinoma and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway renal cell carcinoma across Western Health and Social Care Trust hospital sites resulting in
- Achievement of the renal cell carcinoma 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of renal cell carcinoma patients
- Increase in treatment rates for renal cell carcinoma cell patients
- Optimisation of service delivery
MSD Benefits
- Better understanding of renal cell carcinoma patient needs
- Enhanced reputation of MSD through partnership work
As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD
Job Code GB-NON-07059 | Date of Preparation – February 2023
Renal Cell Carcinoma Southern Health and Social Care Trust Pathway Development Project (PDP)
Project Title
Renal Cell Carcinoma Southern Health and Social Care Trust Pathway Development Project (PDP)
Organisations involved
MSD
Southern Health and Social Care Trust
Summary
A Renal Cell Carcinoma pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all renal cell carcinoma patients receive optimal cancer care. There is an opportunity in Southern Health and Social Care Trust to optimise the renal cell carcinoma pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved renal cell carcinoma pathway and achievement of the renal cell carcinoma 31-day and 62-day Cancer Waiting Time Targets. The project intends to begin on 01/07/22 and anticipates a finish date on 31/12/23.
Project Objectives
The primary objective of this project is the optimisation of renal cell carcinoma pathways across Southern Health and Social Care Trust. Specifically contributing towards; –
- An optimised renal cell carcinoma pathway
- Achievement of the renal cell carcinoma 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets
Project Approach
- Pathway mapping of each renal cell carcinoma service in Southern Health and Social Care Trust and creation of Lucid charts depicting the current pathway
- Gap analysis contributing towards co-creation of service re-design plans from gap analysis outputs for each site managing renal cell carcinoma patients in Southern Health and Social Care Trust
- Implementation of an optimised pathway for each site managing renal cell carcinoma patients across Southern Health and Social Care Trust
- Both parties commit to measuring the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the renal cell carcinoma pathway in Southern Health and Social Care Trust.
- Quicker diagnosis and treatment of renal cell carcinoma and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway in renal cell carcinoma across Southern Health and Social Care Trust hospital sites resulting in:
- Achievement of the Renal Cell Carcinoma 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of renal cell carcinoma patients
- Increase in treatment rates for renal cell carcinoma
- Optimisation of service delivery
MSD Benefits
- Better understanding of renal cell carcinoma patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
Total Project = £6020.48; MSD contribution = £3100; NHS Contribution = £2920.48
Job Code GB-NON-06937 | Date of Preparation – January 2023
Renal Cell Carcinoma Belfast Health and Social Care Trust Pathway Development Project (PDP)
Project Title
Renal Cell Carcinoma Belfast Health and Social Care Trust Pathway Development Project (PDP)
Organisations involved
MSD
Belfast Health and Social Care Trust
Summary
A Renal Cell Carcinoma pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all renal cell carcinoma patients receive optimal cancer care. There is an opportunity in Belfast Health and Social Care Trust to optimise the renal cell carcinoma pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved renal cell carcinoma pathway and achievement of the renal cell carcinoma 31-day and 62-day Cancer Waiting Time Targets. The project intends to begin on 01/07/22 and anticipates a finish date on 31/12/23.
Project Objectives
The primary objective of this project is the optimisation of renal cell carcinoma pathways across Belfast Health and Social Care Trust. Specifically contributing towards;
- An optimised renal cell carcinoma pathway
- Achievement of the renal cell carcinoma 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets
Project Approach
- Pathway mapping of each renal cell carcinoma service in Belfast Health and Social Care Trust and creation of Lucid charts depicting the current pathway
- Gap analysis contributing towards co-creation of service re-design plans from gap analysis outputs for each site managing renal cell carcinoma patients in Belfast Health and Social Care Trust
- Implementation of an optimised pathway for each site managing renal cell carcinoma patients across Belfast Health and Social Care Trust
- Both parties commit to measuring the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the renal cell carcinoma pathway in Belfast Health and Social Care Trust
- Quicker diagnosis and treatment of renal cell carcinoma and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway in renal cell carcinoma across Belfast Health and Social Care Trust hospital sites resulting in:
- Achievement of the Renal Cell Carcinoma 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of renal cell carcinoma patients
- Increase in treatment rates for renal cell carcinoma
- Optimisation of service delivery
MSD Benefits
- Better understanding of renal cell carcinoma patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
Total Project = £8099.42; MSD contribution = £4300; NHS Contribution = £3799.42
Job Code GB-NON-06899. Date of Preparation – January 2023
Lung - Active Projects
NHS Tayside Lung Cancer Pathway Development Project (PDP)
Project Title
NHS Tayside Lung Cancer Pathway Development Project (PDP)
Organisations involved
MSD
NHS Tayside
Summary
A national optimal lung cancer pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all lung cancer patients receive optimal cancer care. There is an opportunity in NHS Tayside to optimise the lung cancer pathway in line with the national optimal lung cancer pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved lung cancer pathway aligned with the national optimal pathway and achievement of the Cancer Waiting Time Targets. The project intends to run for approximately 9 months.
Project Objectives
The primary objective of this project is the optimisation of lung cancer pathways across NHS Tayside. Specifically contributing towards; –
- An optimised lung cancer pathway aligned to the national optimal lung cancer pathway
- Achievement of the Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the lung cancer pathway in NHS Tayside.
- Quicker diagnosis and treatment of lung cancer and hence improving the chance of successful treatment.
NHS Benefits
An optimised pathway in lung cancer across NHS Tayside hospital sites resulting in
- Achievement of the Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of lung cancer patients
- Increase in treatment rates for lung cancer
- Optimisation of service delivery
MSD Benefits
- Better understanding of lung cancer patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD
GB-NON-09373 | May 2024
Kent & Medway Cancer Alliance Pathway Development Project (PDP)
Project Title
Kent & Medway Cancer Alliance Pathway Development Project (PDP)
Organisations involved
MSD
Kent & Medway Cancer Alliance
Summary
A national optimal lung cancer pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all lung cancer patients receive optimal cancer care. There is an opportunity in Kent & Medway to optimise the lung cancer pathway in line with the national optimal lung cancer pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved lung cancer pathway aligned with the national optimal pathway and achievement of the lung cancer 28 day Faster Diagnostic Standard and the 31 and 62 day Cancer Waiting Time Targets. The project intends to run for approximately 12 months.
Project Objectives
The primary objective of this project is the optimisation of lung cancer pathways across Kent & Medway. Specifically contributing towards; –
- An optimised lung cancer pathway aligned to the national optimal lung cancer pathway
- Achievement of the lung cancer 28 day Faster Diagnostic Standard, 31-day treatment target and 62 day referral to treatment Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the lung cancer pathway in Kent & Medway.
- Quicker diagnosis and treatment of lung cancer and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway lung cancer across Kent & Medway hospital sites resulting in
- Achievement of the lung cancer 28 day Faster Diagnostic Standard, 31-day treatment target and 62 day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of lung cancer patients
- Increase in treatment rates for lung cancer
- Optimisation of service delivery
MSD Benefits
- Better understanding of lung cancer patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD
GB-NON-09627 | June 2024
Newcastle NHS Foundation Trust Lung Cancer Pathway Development Project (PDP)
Project Title
Newcastle NHS Foundation Trust Lung Cancer Pathway Development Project (PDP)
Organisations involved
MSD
Freeman Hospital
Royal Victoria Hospital
Summary
There is an opportunity in Newcastle to optimise the lung cancerpathway in line with the national optimal lung cancer pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved lung cancer pathway aligned with the national optimal pathway and achievement of the lung cancer 28 day Faster Diagnostic Standard and 62-day Cancer Waiting Time Targets. The project intends to run for approximately 12 months.
Project Objectives
The primary objective of this project is the optimisation of lung cancer pathways across Newcastle. Specifically contributing towards; –
- An optimised lung cancer pathway aligned to the national optimal timed lung cancer pathway
- Achievement of the lung cancer 28 day Faster Diagnostic Standard and 62 day referral to treatment Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the lung cancer pathway in Newcastle.
- Quicker diagnosis and treatment of lung cancer and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway lung cancer across Newcastle NHS Foundation Trust hospital sites resulting in
- Achievement of the lung cancer 28 day Faster Diagnostic Standard and 62-day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis, and treatment of lung cancer patients
- Increase in treatment rates for lung cancer.
- Optimisation of service delivery
MSD Benefits
- Better understanding of lung cancer patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines.
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD
GB-NON-09322 | April 2024
St Bartholomew’s Hospital Equity of Access to Lung Cancer Treatment For Diverse Communities Pathway Development Programme (PDP)
Project Title
St Bartholomew’s Hospital Equity of Access to Lung Cancer Treatment For Diverse Communities Pathway Development Programme (PDP)
Organisations involved
MSD
Barts NHS Trust- St Bartholomew’s Hospital
Summary
There is an opportunity in Barts NHS Trust to optimise the Lung Cancer Patient Pathway. The aim of the project is to map and understand the lung cancer patient pathway from delivering the diagnosis results to delivery of either standard treatment or inclusion into a clinical trial, with a specific focus on understanding the needs of patients from all communities. This will be achieved through pathway mapping workshops and one to one interviews with all healthcare professionals and other relevant decision makers who are responsible for the patient pathway. The second aim is to address some of the inefficiencies seen within the pathway to ensure equity of all for all treatment options, this will be achieved though GAP Analysis workshops and action planning through the implementation of the project. This project is part of a broader piece of work with St Bartholomew’s hospital to help support improvements in treatment access for diverse communities including uptake of clinical trials. This project will help to inform and direct the interventions needed. The project intends to run for approximately 12 months.
Background
Bart’s Health NHS Trust serves one of the largest, most diverse populations in the UK1. Providing equitable cancer care including access to and inclusion in clinical trials to this diverse cultural and social demographic of patients is a priority for the trust and hospital. This project is part of a wider cross functional project where MSD are supporting St Bartholomew’s hospital to understand and redress this imbalance. The Lead Lung Cancer Clinician feels there is a lack of clarity on the existing lung cancer patient pathway, especially in relation to the different touch points- patient experiences, and the different approaches of clinicians. Consequently it was decided that pathway mapping exercises and workshops would be needed. This will then help to inform the wider and broader piece of work.
Project Objectives
The Primary Objective of this project is the optimisation of the lung cancer patient pathway, contributing towards an increase in the uptake of standard treatment of care and inclusion in clinical trials amongst patients from diverse communities. Key Measures will be to:
- Understand the current patient pathway.
- Understand the resources and existing support in place (for patients & clinicians) surrounding conversations that happen during these consultations.
- Use the data and workshop findings to understand the challenges and obstacles of the patient pathway from a diverse patient perspective and to support further service improvements to help increase access to cancer treatments (including clinical trials) in diverse communities.
- Identify ways to address the challenges and obstacles within the patient pathway to optimise this pathway.
- Outline and reduce some of the inconsistencies in the existing patient pathway.
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of project completion.
Benefits
Patient Benefits:
- Patients are better supported to make informed decisions on their standard treatment of care or clinical trial inclusion irrespective of demographic
- An increase in the proportionate representation of diverse populations across both uptake of standard treatment of care and inclusion in clinical trials
NHS Benefits:
- Increased understanding of the challenges and inefficiencies of the pathway and potential options to further support in optimising the pathway for diverse communities
- Increased uptake of standard treatment of care and inclusion in clinical trials amongst patients from diverse communities
- A clear pathway in relation to how HCPs and patients from diverse communities interact with the system in lung cancer
MSD Benefits:
- Enhanced reputation of MSD through partnership work
- Increased understanding of the lung cancer patient pathway and the impact when treating patients from diverse communities
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines or inclusion into any active clinical trials
Funding & Resources
This project is a shared contribution of time between St Barts NHS and MSD.
References:
- NHS Choices, WeBelong – Barts Health NHS Trust (www.bartshealth.nhs.uk/webelong/), last accessed 04/03/2024
GB-NON-09026 | March 2024
Lung Cancer Healthbot – Greater Manchester Cancer Alliance Self-Referral to Chest X-Ray Symptom Awareness & Activation Project
Project Title
Lung Cancer Healthbot – Greater Manchester Cancer Alliance Self-Referral to Chest X-Ray Symptom Awareness & Activation Project
Organisations involved
- Greater Manchester Cancer Alliance (GM Cancer)
- The Christie NHS Foundation Trust
- MSD UK Ltd
Summary
In 2022 Greater Manchester introduced a Self-Referral Chest X-Ray (SRCXR) service across some of its localities to enable patients who may have worrying respiratory symptoms to have direct access to a chest x-ray. The service has been running well and GM Cancer wishes to ensure more coverage and awareness of the service and thus increase uptake. A campaign in certain communities both out of home and social media could help bring more awareness and coupled with a sustainable method of activating patients to seek help, will aid more patients to attend the SRCXR service. This project will bring together a digital healthbot co-designed with GM Cancer Alliance and the Lung Pathway Board and developed by MSD with an awareness campaign to help more people become aware of potential symptoms of lung cancer and then take help-seeking action to attend the SRCXR service. Attendance rates to the service, reach of and engagement with the awareness campaigns and healthbot will be measured to help understand the impact of the project on the service.
Background
Since July 2022, members of the public registered at a GP in Bury, Heywood, Middleton & Rochdale (HMR) have been able to access a chest x-ray at their local hospital, without having to see a GP, or book an appointment in advance. The scheme not only intends to offer peace of mind for many, who may be experiencing concerns about their health, but to also expose the cases which may need extra attention quickly. Conditions like lung cancer have better outcomes when they are caught earlier[1] , so the pilot intended to improve early diagnosis, and potentially improve outcomes for residents. The initiative is available at select hospitals across the area. GM Cancer acknowledge that the self-referral chest x-ray (SRCXR) service is an effective tool to reach a greater number of patients and thus impact earlier diagnosis rates, positively contributing to the NHS long term plan’s 75% target.
Project Objectives
The primary aim of the project is to provide residents of Bury and HMR localities with a digital tool that will signpost them to local services. The secondary aim of the project is to increase awareness and uptake of the SRCXR service via the co-developed Healthbot and measure its impact. The project will run over 9 months and then be evaluated.
Benefits
Potential Patient Benefits
- Awareness of symptoms relating to lung cancer and awareness of services available
- Patients can validate symptom concerns
Potential NHS Benefits
- Wider awareness of SRCXR service
- Use of a sustainable method to improve patient activation to help seeking behaviour as opposed to telephone calls
- Support the utilisation of SRCXR service
- Potential for increase in disease diagnosis and treatment rates
Potential MSD Benefits
- Testing of the Healthbot in the UK
- Understand the wider use of the tool
- The intended benefits of raising awareness of lung cancer symptoms and encouraging a self-referral to chest x-ray may mean that more patients have access to treatment options in line with NICE/SMC guidance, which may or may not include MSD medicines
Funding
This project is a shared contribution between GM Cancer and MSD. The total project cost is £69,600
GB-NON-08732 | January 2024
References
[1] Cancer Research UK; Survival for Lung Cancer document; last accessed 18th January 2024 Survival for lung cancer | Cancer Research UK
South Yorkshire and Bassetlaw Cancer Alliance Lung Cancer Pathway Development Project (PDP)
Project Title
South Yorkshire and Bassetlaw Cancer Alliance Lung Cancer Pathway Development Project (PDP)
Organisations involved
- MSD
- South Yorkshire and Bassetlaw Cancer Alliance
- Barnsley Hospital Foundation Trust
- Sheffield Teaching Hospital NHS Foundation Trust
- The Rotherham NHS Foundation Trust
- Doncaster and Bassetlaw Teaching Hospitals Foundation Trust
- Chesterfield Royal Hospital Foundation Trust
Summary
There is an opportunity in South Yorkshire Bassetlaw to optimise the lung cancer pathway in line with the national optimal lung cancer pathway to improve the service quality, service efficiency, productivity, and patient experience. The desired outcome of this project is an improved lung cancer pathway aligned with the national optimal lung cancer pathway and achievement of the lung cancer 62-day Cancer Waiting Time Targets. The project intends to run for approximately 12 months.
Project Objectives
The primary objective of this project is the optimisation of lung cancer pathways across South Yorkshire and Bassetlaw Cancer Alliance. Specifically contributing towards; –
- An optimised lung cancer pathway aligned to the national optimal timed lung cancer pathway.
- Achievement of the lung cancer 62-day referral to treatment Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the lung cancer pathway in South Yorkshire and Bassetlaw.
- Quicker diagnosis and treatment of lung cancer and hence improving the chance of successful treatment.
NHS Benefits
An optimised pathway in lung cancer across South Yorkshire and Bassetlaw hospital sites resulting in
- Achievement of the lung cancer 62-day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis, and treatment of lung cancer patients
- Increase in treatment rates for lung cancer.
- Optimisation of service delivery
MSD Benefits
- Better understanding of lung cancer patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines.
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD.
GB-NON-08838 | January 2024
Northern Cancer Alliance lung cancer Getting It Right First Time (GIRFT) implementation Project
Project Title
Northern Cancer Alliance lung cancer Getting It Right First Time (GIRFT) implementation Project
Organisations involved
Northern Cancer Alliance + MSD UK Ltd
Summary
This is a collaborative working project between MSD UK and The Northern Cancer Alliance (NCA). NCA is responsible for overseeing the implementation of 3 selected treatment recommendations from the national lung Getting It Right First Time (GIRFT) report. The Northern Cancer Alliance has also identified improvement of Systemic Anti-Cancer Treatment (SACT) rates, introducing a Stage 2 Multi-Disciplinary Team (MDT) meeting and Inter-Patient Transfer (IPT) rules as priorities.
This project intends to work with the NHS Foundation Hospital Trusts (FTs) in the Northern Cancer Alliance (North Cumbria NHS FT, Northumbria Healthcare NHS FT, Newcastle upon Tyne Hospital FT, Gateshead NHS FT, South Tyneside and Sunderland NHS FT, North Tees and Hartlepool NHS FT, Country Durham and Darlington NHS FT and South Tees NHS FT) to implement the 4 GIRFT recommendations and to implement a new Stage 2 MDT meeting for lung cancer and create and implement new Inter-Patient Transfer (IPT) rules across the region. The project aims to run for approximately 12 months.
Background
The Northern Cancer Alliance has some of the highest incidence of lung cancer in the UK, with lung cancer being the 2nd most common cancer in the region. In the Northern Cancer Alliance, lung cancer accounts for 24.8% of cancer deaths1.
Project Objectives
The Project intends to achieve the following:
- Implementation of 4 GIRFT recommendations (and achievement of the targets involved with each priority):
- >85% radical treatment rate in all FTs for NCSLC stage I-II and PS 0-2
- All FTs to ensure Multimodality treatment recording for Stage IIIA and radical treatment for fit patients
- Radical treatment to start by day 49; Surgery, Thermoablation, Radiotherapy to start by day 16 after decision to treat (DTT)
- All trusts should improve their treatment rates with SACT to achieve greater than 70% treatment for fit patients with advanced NSCLC
- Implementation of Stage 2 MDTs in all FTs across the region
- Agreement and implementation of region wide IPT rules for lung cancer
Benefits Realisation
Benefits/ Impact to patients
- More options for treatment for lung cancer patients
- More rapid access to treatment for lung cancer patients
- Better experience of the healthcare system
Benefits to the NHS partner
- Implementing GIRFT and implementing new ways of working with Stage 2 MDT and IPT ‘rules’
- Cancer Waiting Time performance improvement (28-day, 62 day & 31 day).
Benefits to MSD
- The intended benefits of implementing GIRFT recommendations across NCA, may mean that more patients have access to treatment options in line with NICE guidance, which may or may not include MSD medicines
- Better understanding of the challenges faced by the NHS in delivering high-quality patient services and care
- Reputational benefits of MSD collaborating with the NHS to support patient care
Resources
This project is a shared contribution of time between Northern Cancer Alliance and MSD UK
Ref 1: NHS Digital, Cancer registration statistic’s 2019 (published 2021) North East had the highest rate of cancer incidence for males and females – NDRS (digital.nhs.uk) last accessed 31st May 2023
GB-NON-07314 | Date of Prep May 2023
Manchester University NHS Foundation Trust Patient Experience App
Project Title
Manchester University NHS Foundation Trust Patient Experience App
Organisations involved
Manchester University NHS Foundation Trust
Summary
Wythenshawe Hospital (Manchester University NHS Foundation Trust) have initiated a RAPID (rapid access to pulmonary intervention and diagnosis) pathway for all lung cancer patients. It aims to reduce the time from GP referral to first treatment to 28 days, less than half the current NHS target. While the pathway has demonstrated advantages in time to diagnosis, further evidence is needed to understand the impact of such a fast-track diagnosis on the patient experience. The aim of the project is to work with Manchester University NHS Foundation Trust to develop a patient experience survey. The purpose of the survey is to build an evidence base of what the experience of the patient is within this accelerated pathway, and to facilitate continuous improvement to the pathway through real time analysis.
Background
Wythenshawe hospital have introduced the RAPID program for lung cancer patients, with the aim of 28 day pathway from referral to treatment decision.
While the pathway has demonstrated advantages in time to diagnosis, further evidence is needed to understand the impact of such a fast-track diagnosis on the patient experience.
Currently, patient experience surveys have focused on patients who have been given a cancer diagnosis, this project will address this by gaining insights into all patients going through RAPID- regardless of the diagnosis
Project Approach
- To develop a patient experience app to capture patient satisfaction with the optimal lung cancer pathway within Wythenshawe hospital
- The tool will be co-produced with GM focus group
- The survey will be offered to approximately 500 patients going through the optimal lung cancer pathway at Wythenshawe hospital during a twelve-month period
- Data will be captured and collated, and results reviewed
- Evaluation- App will be built to deliver reports providing data analysis at 3/6/9/12 months. Report will be owned by the NHS and they will give license to MSD to use it. Any publication of data will be approved by both parties
Project Objectives
To work with Wythenshawe hospital to evidence the patient experience whilst in the RAPID (Rapid access to pulmonary assessment and diagnosis) lung cancer pathway.
To share and disseminate that data with the wider lung cancer community, to support the implementation of the NOLCP (or other service improvements) in other Trusts.
Benefits
Benefits to patients:
- For patients currently in RAPID pathway, their patient experience concerns will be identified and addressed
- Addressing patient concerns will continuously improve and develop the service for subsequent patients
- Adding patient experience data to outcome data may prove sufficiently compelling to lead to wider adoption of the pathway in other Trusts, in turn speeding up the pathway there and reducing variation across the NHS
- More rapid diagnoses may improve treatment rates and outcomes for patients
Benefits to NHS:
- Capturing this patient experience data will enable generation of data to demonstrate performance and delivery of the pathway (by monitoring dates of interventions etc.) by the Trust, which may be used for reporting and publication. This will enable them to assess the value of extending the RAPID pathway across wider GM
- Continuously improve the service (e.g. highlighting areas of need or dissatisfaction)
- Provide other hospitals and services evidence to form business cases to adopt the RADID pathway
- Development of a patient experience survey that can be used to support future pathways and projects within the wider NHS, in multiple cancers
Benefits to MSD:
Data generated on the patient pathway and experience prior to treatment decision could be used by MSD for:
- Policy and advocacy activities
- Development of patient support materials
- Advocacy for the RAPID pathway model
- Reputational benefit from partnering on this high profile, innovative project
- All benefits are non-financial and can potentially be realised from the first data read out from the survey
- An opportunity to measure patient experience in other projects in GM and the wider NHS
Funding
People:
From MSD:
- Project manager to attend monthly meetings with steering group, to support and review progress throughout 12-month pilot
- Oncology franchise and medical to advise on the methodology and design of the survey and analytical methods
From the NHS partner:
- Development of App with 3rd party provider
- Identification of suitable patients to take part in the survey, and encouraging usage of survey in 500 plus patients
- Attendance at monthly meetings with steering group
Funds
Total MSD £41,650
Total NHS £37,500
Total project=£79,150
GB-NON-03087 | August 2020 | Re-approved August 2022
Lung - Completed Projects
Hywel Dda University Health Board lung Pathway Development Project (PDP)
Project Title
Hywel Dda University Health Board lung Pathway Development Project (PDP)
Organisations involved
MSD
Hywel Dda University Health Board
Summary
A national optimal lung cancer pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all lung cancer patients receive optimal cancer care. There is an opportunity in Hywel Dda University Health Board (HDUHB) to optimise the lung cancer pathway in line with the national optimal lung cancer pathway to improve the service quality, service efficiency, productivity, and patient experience. The desired outcome of this project is an improved lung cancer pathway aligned with the national optimal pathway and achievement of the lung cancer 62 day Cancer Waiting Time Targets. The project intended to start on 1st April 2022 and was extended to finish on 31st December 2023.
Benefits Realised
Due to significant and unanticipated capacity issues within the HDUHB system which needed to take priority, it has not been possible to complete the outcome summary for the HDUHB lung cancer pathway PDP.
Funding & Resources
This was a project with shared time commitment from HDUHB & MSD
Lessons Learnt
Creation of a formal contingency plan should any of the key stakeholders and MSD project manager have time capacity issues or their organisation suffers significant disruption which affects stakeholder capacity during the implementation of the project.
Publications
No publications are planned.
GB-NON-09771 | July 2024
EPIC: Early Prehabilitation in Lung Cancer
Project Title
EPIC: Early Prehabilitation in Lung Cancer
Organisations involved
NHS Lothian, Edinburgh Cancer Center, South East Scotland Cancer Network (SCAN) & MSD
Summary
The Edinburgh Cancer Centre, NHS Lothian and SCAN worked with MSD to understand the feasibility of utilising prehabilitation techniques in patients with advanced metastatic lung cancer with the overall aim of reducing symptom burden, improving patient fitness and increasing treatment rates for lung cancer.
Lung cancer is the most common cancer in Scotland with more than 5,500 registrations in 20191 and it accounted for over 25%2 of all cancer deaths. Around 50% of patients are identified at stage 41 meaning that access to treatment can be limited and this is compounded by the fact that many patients have comorbidities and are frail which adds to the symptom burden3.
Prehabilitation is the practice of enhancing a patient’s functional and psychological capacity before treatment commences. Ideally, prehabilitation interventions start at diagnosis, helping people to prepare for the next treatment stage in their journey of care.
The project was based at St John’s Hospital, Livingston and aimed to include all patients with locally advanced and advanced lung cancer (visible metastases or mediastinal nodes on diagnostic CT scan). It was run over 30 months including during the covid pandemic which impacted the expected patient numbers.
The principal objectives of the project were to –
- maximise patient fitness as they are investigated and start treatment for lung cancer
- Reduce symptom burden
- Improve nutrition and stop weight loss
- Increase treatment rates for lung cancer
Benefits Realised
NHS Lothian is gathering and analysing the data for this project and have plans to publish the outcomes in early 2024. NHS Lothian has requested the outcomes of this project are published by MSD at the same time as their publication. Therefore, to accommodate this request, MSD plans to publish the outcomes of this project in early 2024, at the same time as NHS Lothian’s publication. This outcome summary will be updated once NHS Lothian and MSD have agreed on a publication date in early 2024.
Funding & Resources
This was a project with shared time commitment from NHS Lothian & MSD
- Cancer incidence in Scotland – Cancer incidence and prevalence in Scotland to December 2019 – Cancer incidence in Scotland – Publications – Public Health Scotland (https://publichealthscotland.scot/publications/cancer-incidence-in-scotland/cancer-incidence-in-scotland-cancer-incidence-and-prevalence-in-scotland-to-december-2019/)
- Scottish – ScotPHO (https://www.scotpho.org.uk/health-conditions/cancer-lung/data/scottish/
- The prevalence of comorbidity in the lung cancer screening population: A systematic review and meta-analysis – PubMed (nih.gov)
GB-NON-08717 | December 2023
University Hospitals Dorset NHS Lung Pathway Development Project (PDP)
Project Title
University Hospitals Dorset NHS Lung Pathway Development Project (PDP)
Organisations involved
University Hospitals Dorset NHS FT (UHD)
MSD-UK
Summary
A national optimal lung cancer pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all lung cancer patients receive optimal cancer care. There was an opportunity in and across Dorset to optimise the lung cancer pathway in line with the national optimal lung cancer pathway (NOLCP) to improve the quality, efficiency, productivity, and patient experience of the service. The project began on 1st June 2021 and finished on 30th January 2023.
Project Objectives
The primary objective of this project was the optimisation of lung cancer service pathway at University Hospital Dorset – Poole. Specifically contributing towards; –
- An optimised or improved lung cancer pathway aligned to the national optimal timed lung cancer pathway.
- Achievement of the lung cancer 2 week wait (2WW), 28 day Faster Diagnostic Standard, 31-day treatment target and 62-day referral to treatment Cancer Waiting Time (CWT) target.
Benefits Realised
Patient Benefits
- Per Getting IT Right First Time (GIRFT) recommendation, a pathway navigator is now embedded in the Oncology Clinical Nurse Specialist (CNS) team to support patients by navigating them through all or parts of the cancer pathway from a referral with suspected cancer to a confirmed cancer diagnosis and so enabling the trust to help sustain meeting the 28-day Faster Diagnosis Standard (GIRFT LUCN2d – All lung cancer teams should have an administrative navigator post integrated into their specialist nursing team)
- Faster diagnosis of lung cancer was improved, and treatment of each patient’s lung cancer has been sustained above national targets (reference Benefits to NHS University Hospital Dorset below)
NHS Benefits
- Pathway navigator embedded in oncology CNS team to improve efficiencies and support 28 days FDS.
- CNS led Outpatient Triage Clinic to free up Respiratory Consultant Time (+ 1-day Full Time Equivalent (FTE)/month)
- Full time lung pathologist recruited to support 5 day-service (consistent with GIRFT recommendation LUC7e)
- Full benefits to be reviewed and analysed as improvement work is still ongoing.
An initial analysis taken from UHD CWT data Jan 2023 to June 2023 (vs Jan 2021 to June 2021) reported the following changes vs the lung cancer pathway targets (Increase/decrease vs Performance Standard):
- 28-day Faster Diagnostic Standard performance improved to 92.1% (+1.3%. Target 75%),
- 2WW performance improved to 99.0% (+1.4% improvement. Target 93%)
- The 31-day wait was 97.96% (-0.65%. Target 96%)
- The 62-day referral to treatment CWT was 50% (+0.98%. Target 85%) – upon discussions with sponsors at the Trust, it is thought the increase was limited due to the extended impact of the pandemic and recent industrial action.
MSD Benefits
- Increased understanding of the lung cancer pathway, the challenges to current service provision and solutions to enable improvement towards the NOLCP target of diagnosis to treatment in 49 days.
- A better understanding of customer and patient needs in lung cancer.
- Enhanced reputation of and legitimacy for partnership work with MSD
- This collaboration may have enabled more patients to access innovative treatments in line with NICE guidance which may or may not have included MSD medicines.
Funding & Resources
This was a project with shared time commitment from University Hospital Dorset – Poole NHS FT & MSD.
Lessons learnt
The importance of:
- Developing a strong sponsor (management, clinical and administrative) engagement plan to aid the Corporate Cancer Manager with the frequent communication required to maintain the momentum and commitment to prioritise pathway improvement activity.
- Senior stakeholder’s activation to sustain support with a clear vision and appetite that is necessary to effect change in clinical services.
- Aligning on a communication plan for disseminating the incremental successes of service change during the project to maintain support to make service development permanent and sustainable.
- Creating of a formal contingency plan should any key stakeholders leave their post or the organisation during the implementation of the project.
- Identification of a trust ‘Project Manager’ resource to support implementation of the action plans from the end of the project through the immediate 6-month period to evaluation.
- NHS governance audit trail – when improvement projects are signed off at senior level, they are added to a project register so that they can be prioritised when resource is limited and there are other operational pressures.
Publications
No publications have been planned.
GB-NON-07838 | October 2023
Manchester Royal Infirmary (MRI) Pathway Development Project (PDP)
Project Title
Manchester Royal Infirmary (MRI) Pathway Development Project (PDP) Outcome Summary
Organisations involved
MSD-UK
Manchester Royal Infirmary
Summary
A national optimal lung cancer pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all lung cancer patients receive optimal cancer care. There is an opportunity in Greater Manchester to optimise the lung cancer pathway in line with the national optimal lung cancer pathway to improve the service quality, service efficiency, productivity, and patient experience. The desired outcome of this project is an improved lung cancer pathway aligned with the national optimal pathway and achievement of the lung cancer 28 day Faster Diagnostic Standard and the 31- and 62-day Cancer Waiting Time (CWT) Targets. The project began in October 2022 and finished in March 2023.
Benefits Realised
Through implementation of MSD’s Pathway Development Programme with Manchester Royal Infirmary Hospitals team, MSD provided project management support to assess the current state of the lung cancer pathway and provided a gap analysis contrasting the pathway with the National Optimal Lung Cancer Pathway. MSD then supported the implementation of improvement initiatives to close these gaps and assisted with the measurement of their impact. As a result of the project, the following benefits were realised:
Patient Benefits
- Quicker diagnosis and time to treatment of lung cancer has been reported through this project potentially leading to improved patient outcomes. Please refer to days saved in NHS benefits below
- Direct upgrades of CXR to CT scan if suspicion of lung cancer
- Electronic alert system from radiologist to lung cancer team via HIVE (new integrated patient electronic record keeping system)
- Introduction of a new lung cancer team member, a Pathway Navigator, which aims to support the streamlined lung cancer investigations and patient appointments throughout the pathway
The following initiatives were identified and implemented to support with optimising the lung pathway at MRI
NHS Benefits
- After the workshops we identified some issues with regards to patient flow and communications amongst teams, utilising the HIVE system. Upon identification, the team went onto work with IT and make some necessary adjustments with regards to departmental communication such as triage lists, patient tracking lists and in basket referrals for suspicious lung cancers
- The unit have now successfully achieved the HOT reporting of CT scans, to ensure that they only receive reporting of scans with suspicious lesions- reducing 3-5 days on pathway
- A successful business case to increase lung CNS team from 2 to 4
- Trust secured a navigator and used the workshops to plan implementation of the role to maximise rapid integration to support patients through the lung pathway
- CT guided biopsy slots for MRI patients has been made accessible for GM – CAD (Greater Manchester Cancer Diagnostic System). Which means accessible and unutilised local slots within the Manchester region were facilitated for Manchester patients and benefitted MRI
- Development of timely discussion at MDT with initial provisional histopathology report by pathologist and final histopathology report before initiation of cancer treatment
- Implementation of local MDT toolkit validated by greater Manchester Cancer Alliance
An initial analysis taken from MRI CWT data 2022- 2023 reported an improvement of the lung cancer pathway targets:
- Data is currently being audited for cancer waiting times and may be included at a later stage
MSD Benefits
- Better understanding of lung cancer patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
- This collaboration may have enabled more patients to access innovative treatments in line with NICE guidance which may or may not have included MSD medicines.
Funding & Resources
This was a project with shared time commitment from Manchester Royal Infirmary & MSD.
Lessons learnt
An agreed strong engagement plan with the respiratory department, with commitment from all departmental members to fully engage and drive this pathway work forward within the NHS contributed significantly to the success of this project.
Publications
There are currently no plans to publish the outcomes of this project by the hospital trust as per the date of this summary.
GB-NON-08222 | October 2023
Glasgow Royal Infirmary lung prehabilitation Pathway Development Programme (PDP)
Project Title
Glasgow Royal Infirmary lung prehabilitation Pathway Development Programme (PDP)
Organisations involved
Respiratory Medicine Department Glasgow Royal Infirmary (GRI)
MSD-UK
Summary
The aim of this project was to map the lung cancer treatment and prehabilitation service pathways, identify service inefficiencies and facilitate service improvement. The purpose of which was the improvement of pathway service quality, efficiency, and productivity. At an early stage, and because of the parallel development of a national optimal lung cancer pathway, the lead requested we support a similar piece of work to gain insights for all lung cancer MDTs across Scotland. Because of this request, we entered into a separate piece of pathway work in collaboration with the UK Lung Cancer Coalition (UKLCC) which aims to improving health guidance in relation to lung cancer. This resulted in the narrowing of the focus of this project to only the prehabilitation pathway at the GRI. This project began June 2022 and finished November 2022.
Benefits Realised
Through implementation of MSD’s Pathway Development Programme (PDP) with the Glasgow Royal Infirmary team, MSD provided project management support to assess the current state of the lung prehabilitation cancer pathway and provide a gap analysis of the available patient support services on the ability to provide robust prehabilitation to their patients. As a result of the project, the following benefits were realised:
Patient Benefits:
- Improved referral pathway to general prehabilitation patient support services in Glasgow. Before this project, there was no pathway set up for healthcare professionals to refer to prehabilitation services and services available were not widely known across GRI
- Access to prehabilitation support aims to maintain fitness levels, nutrition, and mental wellbeing. This is yet to be measured by GRI
NHS benefits:
- Creation of a 1-year physiotherapy led lung cancer prehabilitation service improvement project based at the Beatson cancer centre that will benefit patients from GRI, Gartnavel General Hospital, and the New Victoria Hospital. This service hasn’t started yet, therefore no data available at time of the outcome summary publication
- Improved Healthcare Professional understanding of limited scope of currently available general prehabilitation patient support services in Glasgow. This is to be measured by GRI at a later date
- Impact on treatment rates for lung cancer was not measured or used as a baseline as the project scope changed
MSD Benefits:
- Better understanding of lung cancer patient needs
- Enhanced reputation of MSD through partnership work. Four NHS stakeholders completed a survey following the PDP and the results are shown below:
- Average score of 9/10 and 6.75/10 was scored for being satisfied with MSD’s collaborative working project and believed MSD contributed to their organisation’s cancer service pathway respectively
- Average score of 9.5/10 would recommend working in collaboration with MSD to others
- Quotes taken from the survey when questioned, “what has improved in your service/pathway following this collaborative working project” from NHS Stakeholders:
- “Better understanding of deficiencies and needs, particularly regarding prehab”
- “Highlighted already existing resources we were not using or not using fully and from a starting point of having a vague idea what we wanted to improve, we learned a great deal and can now see a clear path to achieving desired improvement and how that should look”
- When questioned on feedback of your experience of working in collaboration with MSD
- “Very supportive and enthusiastic”
- “This has been an eye opener in many ways – several projects originated and gained momentum once we started to collaborate – from general service prehab design to specific trial at Beatson, to a Scotland wide gap analysis for the National Lung Cancer Pathway, and hopefully further collaboration to implement findings in a local pilot but with view to regional improvements. It was difficult to find time sometimes but well worth it”
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their SMC approved medicines
Funding & Resources
This was a project with shared time commitment from Glasgow Royal Infirmary lung cancer team & MSD
Lessons learnt
Committed project manager and communication plan is essential to ensure all relevant parties are kept informed
Strong clinical leadership with a clear vision for change is necessary to effect change
A longer time period is required to make and measure significant service improvements
Publications
There are currently no plans to publish the outcomes of this project by the hospital as per the date of this summary
GB-NON-07558 | October 2023
Belfast Health and Social Care Trust Pathway Development Project (PDP)
Project Title
Belfast Health and Social Care Trust Pathway Development Project (PDP)
Organisations involved
Belfast Health and Social Care Trust & MSD UK
Summary
The aims of this project were to optimise the lung cancer pathway across the Belfast Health and Social Care Trust through service redesign with the lung cancer multidisciplinary team and focussing on the pathway from red flag referral through to first definitive treatment. Specifically, contributing towards achievement of the lung 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets (CWT). MSD helped to project manage the implementation of this initiative through MSD’s Pathway Development Programme. The project started in March 2022 and finished December 2022
Benefits Realised
Through implementation of MSD’s Pathway Development Programme with the Belfast Health and Social Care Trust team, MSD provided project management support to assess the current state of the lung cancer pathway and provide a gap analysis contrasting the pathway with the National Optimal Lung Cancer Pathway. MSD then supported the implementation of improvement initiatives to close these gaps and assisted with the measurement of their impact. As a result of the project, the following benefits were realised:
Patient Benefits
- Optimisation of the lung cancer pathway which allows patients to navigate through the pathway more efficiently potentially increasing the chance of a successful outcome if patients are diagnosed earlier on in the pathway. Refer to NHS benefits below to see number of days potentially saved.
NHS Benefits
- Pathology – Implementation of Next Generation Sequencing (NGS) to create a suite of reflex molecular testing to avoid the need to send samples to reference centres reducing sample turnaround time by a minimum of 4 weeks. This will benefit all lung cancer patients diagnosed in Northern Ireland (approx. 1355/ annum). NGS currently achieving a 91% turnaround within 10-14 days.
- Fast track histopathology pathway to expedite patient samples within 24 hours of the multi-disciplinary meeting (MDM), reducing deferrals and optimising the pathway by 8 days and benefitting approx. 190 patients per year.
- New transfer pathway between histopathology and molecular pathology optimising the pathway by 3 days per sample.
- Interventional radiology – optimised pathway reducing the time to fine needle biopsy from 8 weeks to 4 weeks benefiting approx. 120 patients per year who will have a reduction of 4 weeks to have their fine needle biopsy.
- Establishment of a Computerised Tomography (CT) rapid access pilot which is assessing the impact of fast-tracking definite lung cancer patients (confirmed by Chest X-Ray) to a CT scan within 5 days vs current 14 days.
- Cost per case -new guidance for consultants to start patients’ treatment without delay and allow Cost per Case documentation to be completed in parallel. The new guidance is anticipated to reduce the time to initiation of treatment by approximately 1-2 weeks.
- Additional MDM tracker recruited to provide cover and additional capacity to track patients through the lung cancer pathway to expedite patients faster and monitor potential areas of delays.
- Overall treatment rates were not measured as part of this Pathway Development Programme
MSD Benefits
- Better understanding of lung cancer patient needs
- Enhanced reputation of MSD through partnership work. Six NHS stakeholders completed a survey following the PDP and the results are shown below:
- Average score of 9.5/10 and 8.5/10 was scored for being satisfied with MSD’s collaborative working project and believed MSD contributed to their organisation’s cancer service pathway respectively.
- Average score of 8.33/10 would recommend working in collaboration with MSD to others.
- Quotes taken from the survey when questioned, “what has improved in your service/pathway following this collaborative working project” from NHS Stakeholders:
- “Expedited CT pathway, expedited pathology sample delivery and inception of Next Generation Sequencing”
- “Improving care for our patients”
- “Improved communication across specialities”
- When questioned on feedback of your experience of working in collaboration with MSD
- “Great team to work with, very engaging and encouraging. A sense that they were very much invested in patient benefit”
- “MSD gave us additional project management capacity and an external voice which was really helpful”
- “Excellent experience – without their focus we would have had difficulty achieving what we have”
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE approved medicines
Funding & Resources
This was a project with shared time commitment from both the NHS & MSD
Lessons learnt
- Communication is key between all parties which is especially important between departments to optimise decision making and establish the appropriate intervention for the patient as early as possible
- Regular touch points to ensure clarity of expectation and outcomes
- Clear plan of action and review within stakeholder meetings
- Despite the best efforts of the NHS and MSD to optimise the lung cancer patient pathway, attainment of the Cancer Waiting Time (CWT) targets remains challenging. The CWT performance for lung cancer has remained steady against a backdrop of decreasing performance for other tumour types suggestive of even greater challenges within the system.
- 31–day CWT compared Jan-Mar 2021 vs Jan-Mar 2023: 87% vs 84%
- 62-day CWT compared Jan-Mar 2021 vs Jan-Mar 2023: 50% vs 50%
Publications
There are currently no plans to publish the outcomes of this project by the hospital trust as per the date of this summary.
GB-NON-07574 | Date of Preparation: July 2023
Peninsula Cancer Alliance Lung Cancer Pathway Development Project (PDP)
Project Title
Peninsula Cancer Alliance Lung Cancer Pathway Development Project (PDP)
Organisations involved
Peninsula Cancer Alliance; Royal Devon & Exeter Hospital; University Hospital Plymouth; North Devon Hospital; Royal Cornwall Hospital and Torbay & South Devon Hospital
MSD-UK
Summary
A national optimal lung cancer pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all lung cancer patients receive optimal cancer care. There was an opportunity in Devon and Cornwall to optimise the lung cancer pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project was an improved lung cancer pathway aligned with the national optimal pathway and achievement of the lung cancer 28 day Faster Diagnostic Standard (FDS) and the 62-day Cancer Waiting Time Target. The project began on 3rd January 2022 and finished on 30th November 2022
Each provider Trust completed the 28-day lung FDS pathway audit indicating the number of pathway days that a representative group of patients took to reach each pathway milestone, as defined by the NHSE 28-day best practice timed pathway. The audit results were analysed and feedback meetings were held with each provider Trust to discuss challenges and potential pathway solutions to improving attainment of the 28-day FDS cancer wait time target. As a result, each of the provider Trusts implemented different improvement initiatives and the results were measured by comparing the number of 28-day breaches (NHSE data) for the period June to September, versus the baseline period of January to April 22
Benefits Realised
-
Patient Benefits
- As a result of the audit and improvement work, patients benefited from a faster diagnostic pathway with fewer pathway times breaching the standard overall, please see NHS benefits
-
NHS Benefits
- Royal Cornwall Hospital introduced a new pathway to triage CT scans on the same day that they were performed and prior to the outpatient appointment
- Lung patient breaches of the 28-day FDS reduced from 18% down to 10%*
- University Hospital Plymouth increased their workforce to support improvements in the pathway through employing a Cancer Improvement Facilitator, a Lung CNS and a trainee Advanced Nurse Practitioner
- Lung patient breaches of the 28-day FDS reduced from 17% down to 9%*
- Torbay & South Devon Hospital focussed improvement work on developing the Radiology service to prioritise urgent referrals from primary care
- The lung patient breaches of the 28-day FDS reduced from 19% down to 18%*
- We did not measure the direct impact on treatment rates in this project
- Royal Cornwall Hospital introduced a new pathway to triage CT scans on the same day that they were performed and prior to the outpatient appointment
*Data obtained from NHSE Cancer Waiting time data. Time periods compared the 4 months June-Sept 2022 against a baseline of Jan-Apr 2022
-
MSD Benefits
- Better understanding of lung cancer patient needs
- Enhanced reputation of MSD through partnership work. NHS stakeholder commented their experience of working in collaboration with MSD was “very professional, had good subject knowledge and a great support for the Alliance and the wider organisations”
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding & Resources
This was a project with shared time commitment from both the NHS & MSD
Lessons learnt
Effectively engaging a wider group of relevant stakeholders is essential to drive maximum involvement and benefit from improvement work
Publications
At the time of writing this summary there were no plans to publish this data
GB-NON-07092 | June 2023
University Hospitals of Birmingham (UHB) Lung Cancer Pathway Development Project (PDP)
Project Title
University Hospitals of Birmingham (UHB) Lung Cancer Pathway Development Project (PDP)
Organisations involved
University Hospitals Birmingham Foundation Trust (UHB), West Birmingham & Sandwell Foundation Trust, Walsall Foundation Trust, Birmingham & Solihull Integrated Care System (BSOL ICS), Birmingham Health Partners (BHP), Black Country & West Birmingham Strategic Transformational Partnership (BC & WB STP) & West Midlands Cancer Alliance
Summary
A national optimal lung pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all lung cancer patients receive optimal cancer care. There was an opportunity in Birmingham and the referring trusts of the West Midlands to optimise the lung cancer pathway in line with the national optimal lung cancer pathway to improve the service quality, service efficiency, productivity, and patient experience. The desired outcome of this project was an improved lung cancer pathway aligned with the national optimal lung cancer pathway and achievement of the lung cancer 28 day Faster Diagnostic Standard, the 31, 62- and 104-day Cancer Waiting Time Targets. The project began on 1st January 2022 and finished on 23rd May 2022. The data set used for baseline and impact evaluation was the Milestone Dashboard created by UHB for the project (data collected from September 2022 – Feb 2023).
Project Objectives
The primary objective of this project was the optimisation of lung cancer pathways across Birmingham and referring centres across the West Midlands. Specifically contributing towards; –
- An optimised lung cancer pathway aligned to the national optimal lung cancer pathway
- Achievement of the lung cancer 28 day Faster Diagnostic Standard, 31-day treatment target, 62-day referral to treatment and 104-day Cancer Waiting Time targets
Benefits Realised
Benefits to the Patient
- Delivering Targeted Lung Health Checks (TLHC) screening with trust led screening reviews & protocols
- By the introduction of a new pan-trust prehabilitation & smoking cessation service, it is understood that quicker diagnosis and treatment of lung cancer has been achieved, hence improving the chance of successful treatment. Data is still being collected on this
- By implementing changes, it is understood that there would be an improved patient experience, across the lung cancer pathway in Birmingham and referring centres across the Midlands
NHS Benefits
An optimised pathway in lung cancer across UHB hospital sites resulting in
- Achievement of the 28 day Faster Diagnostic Standard across 3 of 4 sites, 31-day treatment target all sites, 62-day referral to treatment all sites in Cancer Waiting Time targets. Time constraints, industrial action & staff absences meant that the 104 data was unable to be fully verified, so not included at this time, it is planned to be included in a case study
- Through the introduction of new services such as prehabilitation and smoking cessation and by creating dedicated outpatient appointments and capacity for lung resections, it is believed this will enable earlier referral, diagnosis and treatment of lung cancer patients. Data is currently being collected on the impact of this
- Created engagement & collaboration across previously siloed departments
- Optimisation of service delivery is ongoing post the project close
- The rigour & vigour of qualitative & quantitative data enabled efficient & effective change
- Live changes to working practices from the workshops saw the immediate change and benefits. This has led to:
a. a new triage process immediately reducing CWTs by 7 days in 3 sites
b. CNS capacity saving by 1.5 FTE
c. Training on internal data systems for administrative staff, as well as CNS Somerset optimisation training reduced CWTs between departments.
MSD Benefits
- Better understanding of lung cancer patient needs
- Anecdotal enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE approved medicines
Funding & Resources
This was a project with shared time commitment between University Hospitals of Birmingham and MSD. Implementation of improvements resourced solely by UHB.
Lessons learnt:
- Early engagement & inclusion of all players across the pathway, delivered clear insight & understanding of both the issues, as well as the possible solutions.
- Solutions were consciously developed to not put pressure on other parts of the pathway
- Created engagement & collaborative working across previously silos departments
- The exceeded expected uptake of the TLHC increased demand on capacity more than expected
- Not all opportunities were realised and to have done so may have improved the CWTs & pathway improvement further
Publications
No planned publications
GB-NON-07496 | May 2023
Gloucestershire AHP Prehabilitation Service (GAPS) for Lung Cancer Patients
Project Title
Gloucestershire AHP Prehabilitation Service (GAPS) for Lung Cancer Patients
Organisations involved
Gloucestershire Hospitals NHS Foundation Trust (GHNHSFT) and MSD
Summary
We supported Gloucestershire Hospitals NHS Foundation Trust to set up and run a lung cancer prehabilitation service pilot over a 6-month period. We set out to determine the extent to which prehabilitation support for lung cancer patients can either maintain or improve their fitness levels and suitability for systemic anti-cancer therapy (SACT). Secondarily, we intended to measure the effect of prehabilitation on supporting a patient’s physical function and mental resilience. Patients were referred by a lung physician or lung cancer clinical nurse specialist at the diagnostic clinic stage as soon as a lung cancer is highly suspected. They were then assessed for the level of prehabilitation support that would best suit their individual needs and circumstance. We intended to use the evaluation to inform a business case that will seek to ensure recurrent funding and sustainability of this service. MSD provided funding, project management and evaluation support for this initiative. The project began on the 2nd May 2022 and finished on the 30th November 2022.
Benefits Realised
Benefits to the Patient
- As a result of engaging with GHNHSFT ‘s prehabilitation service, 80% of patients either maintained (41 patients or 68%), or improved (7 patients or 12%) their fitness from the time they were referred, through to the time that their treatment started:
- Of the 25 patients measured as PS1 on referral, 16 (64%) maintained their PS1 and 3 (12%) had improved to PS0 by the time of decision to treat
- Of the 27 patients measured as PS2 on referral, although 21 (78%) patients maintained their PS2, 5 (19%) had improved to PS1 by the time of decision to treat
- Patient experience and outcomes of receiving the service were measured:
- 90% of patients stated they were satisfied with the information and advice provided (59% ‘very satisfied’ and 31% ‘satisfied’). This number increased to 97% among those who attended face to face prehabilitation
- 79% of patients reported prehabilitation as improving their overall health and wellbeing either ‘a great deal’ (39%), or ‘a fair amount’ (40%). This number increased to 85% among those attending face to face
- Of the patients that were able to use the psychological skills training, 82% stated that it helped reduce their worry/anxiety either ‘a great deal’ 31% or a fair amount 51%
- Patient’s lifestyle factors were seen to improve as a result of receiving prehabilitation support
- 69% of patients reported that prehabilitation has helped them increased their physical activity
- 57 % reported that prehabilitation has supported a positive change in their diet and nutrition
- 61% of patients stated that prehabilitation supported their emotional well-being during a traumatic time in their lives
Benefits to GHNHSFT
- The development of a prehabilitation service in Gloucestershire dedicated to the needs of lung cancer patients
- Redesign and expansion of the lung cancer patient pathway to incorporate prehabilitation services
- Achievement of recommendations to provide prehabilitation within NHSE Diagnostic Standards of Care for suspected lung cancer
- A service evaluation has been produced highlighting the benefits of a prehabilitation service for lung cancer patients, which can serve as supporting business justification for longer term funding of the service
- The data available through the service evaluation is not sufficient enough to conclude that introducing prehabilitation results in an improvement of treatment rates
Benefits to MSD
- Enhanced reputation of MSD through partnership work
- A better understanding of how prehabilitation services support the needs of lung cancer patients
- An opportunity for MSD to support a service evaluation to help establish the benefits of prehabilitation for lung cancer patients
- An opportunity to engage as a partner with NHS Gloucestershire rather than just being seen as a supplier of medicines to the healthcare system
- Involvement in a potentially scalable service that could be shared with other cancer centres across the UK
Funding & Resources
This project was a shared funding commitment from GHNHSFT and MSD. The total project cost was £28,119
Lessons learnt:
- This project benefitted from having a data lead who can dedicate time to supporting the project through capturing service level data and synthesising the data to produce relevant insights.
- Outlier events such as global pandemics can’t be predicted, but where there is solid justification for setting up a new service, the strength of the case to do so will outlast external challenging forces
- Our forecast of patient numbers referred to the service over the 6-month pilot were affected by several factors (150 forecasted, 76 actual). These were, relying on an average number of patients flowing through the service, not factoring in patients declining the service, clinicians not referring patients who are PS3 and above to the service (due to the likely rapid deterioration and subsequent palliation), as well as perhaps more broadly communicating the service prior to it starting.
- Aligning all constituent elements of a prehabilitation service can be challenging and subject to change. For example, the local smoking cessation service initially agreed to attend prehabilitation sessions, but sadly did not engage when it was up and running.
Publications
Gloucestershire Hospitals NHS Foundation Trust intend to publish the results in a Professional Journal
GB-NON-07465 | May 2023
Nottingham University Hospital NHS Trust Lung Cancer Pathway Development Project (PDP)
Project Title
Nottingham University Hospital NHS Trust Lung Cancer Pathway Development Project (PDP)
Organisations involved
Nottingham University Hospital (NUH) NHS Trust and MSD-UK
Summary
The objective of this project was to improve Nottingham University Hospitals lung cancer service efficiency, service quality, productivity, and patient experience. The desired outcome was an improved lung cancer pathway aligned with the national optimal lung cancer pathway and achievement of the lung cancer 28 day Faster Diagnostic Standard, the 31-day treatment target and 62-day referral to treatment Cancer Waiting Time (CWT) Targets. The project began on 25th July 2022 and finished on 30th November 2022.
Benefits Realised
Through implementation of MSD’s Pathway Development Programme with the Nottingham University Hospitals team, MSD provided project management support to assess the current state of the lung cancer pathway and provide a gap analysis contrasting the pathway with the National Optimal Lung Cancer Pathway. MSD then supported the implementation of improvement initiatives to close these gaps and assisted with the measurement of their impact. As a result of the project, the following benefits were realised:
Patient Benefits
- Quicker diagnosis and time to treatment of lung cancer has been reported, as demonstrated below, through this project potentially leading to improved patient outcomes
- All patients now have access to the Nottingham Education Materials for smoking cessation, diet, activity/exercise, and psychological support which was not previously available. It is envisaged this will support an improved patient experience
NHS Benefits
An initial analysis taken from NUH CWT data Jul 2022-Feb 2023 reported an improvement of the lung cancer pathway targets (Percentage absolute change):
- 28-day Faster Diagnostic Standard improvement of 6.9%
- 2WW improvement of 4.7% (with the 2WW percentage at 100% in Feb 2023)
- 31-day treatment target improvement of 1.5%
- The 62-day referral CWT target is an ongoing measurement as only data to Feb 2023 was available at the time of the outcome summary publication. NUH are continuing to monitor the impact of the project and CWT data
MSD Benefits
- Better understanding of lung cancer patient needs
- Enhanced reputation of MSD through partnership work. NHS stakeholder commented their experience of working in collaboration with MSD was very insightful and helpful to have an objective review
Funding & Resources
This was a project with shared time commitment from Nottingham University Hospital NHS Trust & MSD
Lessons learnt
Development of a strong engagement plan with the pathway manager and regular communication is needed to maintain the project momentum and drive the pathway work within the NHS.
Strong leadership with a clear vision and appetite for change is necessary to effect a change in clinical services.
A communication plan for disseminating the success of the service is essential to gain support to make service development changes sustainable and permanent.
Publications
There are currently no plans to publish the outcomes of this project by the hospital trust as per the date of this summary
GB-NON-07433 | May 2023
Salisbury Lung Cancer Prehabilitation Pathway Development Project (PDP)
Project Title
Salisbury Lung Cancer Prehabilitation Pathway Development Project (PDP)
Organisations involved
Salisbury NHS Foundation Trust and MSD
Summary
A national optimal lung cancer pathway has been created with the intention of improving patient experience and outcomes. In association to an optimised clinical pathway, a prehabilitation service for cancer patients is seen as an effective support intervention to prepare a cancer patient for treatment by maintaining or improving their fitness levels, nutrition, and mental wellbeing. There is an opportunity in Wiltshire to optimise the lung cancer pathway and prehabilitation service to improve service quality and patient experience. The primary objective and desired outcome of this project is an improved lungcancerpathway and prehabilitation service.
Benefits Realised
Patient Benefits
- An improved experience of the lung cancer pathway and prehabilitation services in Wiltshire
- Prehabilitation support to maintain fitness levels, nutrition and mental wellbeing to be best prepared for treatment
- Patient benefits were not fully realised as this project was not completed.
NHS Benefits
- An optimised pathway in lung cancer and prehabilitation service across Wiltshire
- Service map developed of the current prehabilitation service.
- Insights and recommendations report provided to inform future service development initiatives
- Potential for a less fragmented prehabilitation service
- NHS benefits were not fully realised as this project was not completed
MSD Benefits
- Better understanding of how prehabilitation service support the needs of lung cancer patients
- Enhanced reputation of MSD through partnership work
- MSD benefits were not fully realised as this project was not completed
Funding & Resources
This was a project with shared time commitment from Salisbury NHS Foundation Trust and MSD
Lessons learnt
Creation of a formal contingency plan should any of the key stakeholders leave their post or their organisation during the implementation of the project
Publications
No publications are planned
GB-NON-07332 | April 2023
Oxford Respiratory Early Diagnosis Service (REDS) Lung Cancer Pathway Development Project (PDP)
Project Title
Oxford Respiratory Early Diagnosis Service (REDS) Lung Cancer Pathway Development Project (PDP)
Organisations involved
Oxford University NHS Hospitals Trust and MSD-UK
Summary
The objective of this project was the optimisation of lung cancer pathways across Oxfordshire through service redesign with the Oxford REDS team and focussing on early diagnosis through the provision of next day CT scans, outpatient appointments and implementation of diagnostic bundles for patients found to have abnormal chest X-rays. Specifically contributing towards achievement of the lung cancer 28-day Faster Diagnostic Standard, 31-day treatment target and 62-day referral to treatment Cancer Waiting Time target. MSD helped to project manage the implementation of this initiative through MSD’s Pathway Development Programme. The project began on the 6th of October 2020 and finished on the 4th of July 2022
Benefits Realised
Through implementation of MSD’s Pathway Development Programme with the Oxford REDS team, MSD provided project management support to assess the current state of the Oxford early diagnosis service and provide a gap analysis contrasting the current state assessment with the desired ‘to be’ state of next day CT clinics. MSD then supported the implementation of improvement initiatives to close these gaps and assisted with the measurement of their impact. The project achieved the development of next day CT clinics for patients with abnormal chest x-ray alongside hot reporting of the CT scan, a same day outpatient appointment and diagnostic bundling of test ordering on the same day to speed up diagnosis and time to treatment. As a result, the project delivered an improvement in the lung cancer pathway aligned with the NOLCP and achievement of the lung cancer 28-day Faster Diagnostic Standard, as well as the 31 and 62-day Cancer Waiting Time targets.
Benefits Realised
-
Patient Benefits
- Patients experienced an overall average reduction of 16 days in the time taken to move from Chest X-ray to receiving treatment
- Patients expressed an overall satisfaction of the new REDS lung cancer pathway (77% very satisfied and 23% satisfied)
-
NHS Benefits
- An optimised pathway in lung cancer across Oxfordshire hospital sites aligned to the National Optimal Lung Cancer Pathway
- The pathway time from urgent 2ww referral to patients being diagnosed has been reduced on average by 15 days
- 16-day efficiency saving in the average time from Chest X-ray reported to the patient receiving treatment
- We did not measure the direct impact on treatment rates as a result of this project
-
MSD Benefits
- Better understanding of lung cancer patient needs
- Enhanced reputation of MSD through partnership work
Funding & Resources
This was a project with shared time commitment from both the NHS & MSD
Lessons learnt
Strong clinical leadership with a clear vision for change is necessary to effect a change in clinical services
A communication plan for disseminating the success of the service is essential to gain support to make service development changes sustainable and permanent
Publications
There is a tentative plan to publish the outcomes of this project but as of the date of this summary, this has yet to be confirmed.
GB-NON-06512 | January 2023
Northern Cancer Alliance Lung Pathway Board Project
Project Title
Northern Cancer Alliance Lung Pathway Board Project
Organisations involved
Northern Cancer Alliance & MSD UK Ltd
Summary
The Northern Cancer Alliance want to establish a Lung Cancer Pathway Board. The aim of the Lung Cancer Pathway Board is to improve lung cancer care for patients in the North East and North Cumbria, delivering an integrated care pathway from presentation and diagnosis through to personalised and palliative care for lung cancer thus reducing inequalities and delivering improved outcomes.
Background
The Northern Cancer Alliance population is about 5.9% of the England population but has 6.5% of all malignancies and 8.2% of total lung cancers.1
For both males and females, lung cancer is the second most common cancer in the geographies across the Northern Cancer Alliance.1
Lung cancer is the most common cause of cancer death in England, accounting for almost 21% cancer deaths (2017).1
In the Northern Cancer Alliance, lung cancer deaths equate to 24.8% of cancer deaths.1
Project Approach
The role of MSD UK in this project will be to provide a project manager who will provide project management support to the Northern Cancer Alliance Lung Pathway board. The resource required and commitment to complete this project is a project manager for 2 days per week for 12 months.
The Northern Cancer Alliance will provide a clinical chair for the Lung Pathway Board, administrative support and clinical and management supervision for the duration of the project.
Project Objectives
The primary objective of the Lung Cancer Pathway Board is to ensure that every lung cancer patient has access to an equitable, effective and responsive service compliant with National and Local standards, including specific lung cancer waiting times, with the aim of improving outcomes to be in line with world class services. This will include:
- Implementation of the National Optimal Lung Cancer Pathway (NOLCP)
- Implementation of the 28-day Faster Diagnostic Standard (FDS) Timed Lung cancer pathway, addressing local variation and inequality
- Dissemination and adoption of the North East Lung Case Finding Pilots (COPD annual review)
- Streamlining of MDT meetings and developing standards of care for lung cancer pathway
Benefits
Patients
- Earlier detection, diagnosis and treatment of lung cancer patients
- Patients detected, diagnosed and treated at an earlier stage of lung cancer have a greater chance of successful treatment, greater survival rates and clinical outcomes
- Improved patient experience of lung cancer pathway
NHS
- Implementation of the National Optimal Lung Cancer Pathway
- Achievement of lung cancer treatment targets (28-day target, 62 day target)
- Increased percentage of lung cancer patients being diagnosed at stage 1+2 as per the NHS target of 75% by 2028
- Earlier detection, diagnosis and treatment of lung cancer patients leads to more cost-effective use of healthcare resources and increased patient survival and patient experience
MSD
- Increased understanding of the lung cancer pathway and the challenges and solutions to its successful implementation and improvement which can be shared both internally within MSD UK and externally with NHS stakeholders outside of the North East and North Cumbria
- As a pharmaceutical manufacturer of oncology medicines, MSD may see an increase in usage of its NICE approved lung cancer medications as a result of this projects implementation of the National Optimal Lung Cancer Pathway
- Enhanced reputation of MSD UK and the wish to work in partnership further
- MSD UK gains a better understanding of customer and patient needs in lung cancer
Funding
This project involves a pooling of skills and resources between the Northern Cancer Alliance and MSD UK over 12 months.
MSD Contribution = £28,000
NHS Contribution = £36,000
Total Project = £64,000
Update September 2020: The project was put on hold for 3 months in 2020 due to delays caused by COVID-19. It has been agreed to extend the project to March 2021. There will be no further costs or resources required, other than those previously agreed in the initial PID for this project.
References –
- Data supplied by Northern Cancer Alliance
GB-NON-03702 | October 2022
Pan Tumour - Active Projects
Western Health and Social Care Trusts Chemotherapy Day Unit Pathway Development Programme (PDP)
Project Title
Western Health and Social Care Trusts Chemotherapy Day Unit Pathway Development Programme (PDP)
Organisations involved
MSD
Western Health and Social care trusts
Summary
There is an opportunity in Western Health and Social Care trusts to optimise the Chemotherapy Day Unit pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is improvement of the Chemotherapy Day Unit patient pathway and of the Pan Tumour 31-day Cancer Waiting Time Target. The project intends to run for approximately 12 months.
Project Objectives
The primary objective of this project is the optimisation of the Chemotherapy Day Unit Pan Tumour pathways across Western Health and Social Care Trust. Specifically contributing towards; –
- Improving the 31-day treatment target Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the Chemotherapy Day Unit pathways in the Western Health and Social Care Trust
- Faster time to treatment pan tumour and hence improving treatment options
NHS Benefits
Optimised Pan Tumour pathways in the Chemotherapy Day Unit across Western Health and Social Care trust hospital sites resulting in
- Smoother and Quicker time to treatment process following treatment decision
- Potential to improve the pan tumour 31-day cancer waiting time target
- Optimisation of service delivery through the Chemotherapy Day Unit
MSD Benefits
- Better understanding of chemotherapy patient journey through the Chemotherapy Day Unit and patients requiring hospital admission
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD
GB-NON-09634 | July 2024
RM Partners SACT (Systemic Anti-Cancer Therapy) capacity, demand and utilisation project
Project Title
RM Partners SACT (Systemic Anti-Cancer Therapy) capacity, demand and utilisation project
Organisations involved
RM Partners
MSD
Croydon Health Services NHS Trust
Chelsea and Westminster Hospital NHS Foundation Trust
Epsom and St Helier University Hospitals NHS Trust
Kingston Hospital NHS Foundation Trust
London Northwest University Healthcare NHS Trust
Imperial College Healthcare NHS Trust
St George’s University Hospitals NHS Foundation Trust
The Hillingdon Hospitals NHS Foundations Trust
The Royal Marsden NHS Foundation Trust
Summary
The aim of this project is to gain insights into the capacity and demand within the SACT delivery sites across RM Partners cancer alliance using MSD’s capacity insights tool (CIT*) and support optimisation of cancer treatment services to ensure timely access to optimal treatment. The insights from the tool will highlight gaps within their current and future SACT delivery services. Facilitated capacity workshops (led by MSD) will include a review of the report which summarises the insights generated and possible scenarios to allow actionable improvement activities to be identified (by the SACT delivery sites and cancer alliance lead) in order to address any SACT delivery service gaps. These improvement activities will then be pulled through into an action plan which will be implemented by the individual SACT delivery sites. The intent is for SACT delivery service capacity to improve (measured via a second report from CIT) and therefore allow for more timely access to optimal treatments across the cancer alliance.
*CIT – The Capacity Insights Tool is a directional tool to support SACT delivery sites to understand how their capacity (in terms of chairs and workforce) can meet the patient demand on the service now and in the future. The tool can model future scenarios to illustrate how growing demand will impact the SACT delivery service and model the impact of potential changes. The report provided as a result of the tool can be used to identify which potential changes are needed to future proof the service.
Background
- SACT delivery sites across the country are being challenged with increased demand stemming from increasing cancer patients, increasing SACT treatments, novel immunotherapy and combination treatments plus more treatments being delivered in the ambulatory setting. This increased demand for capacity plus workforce pressures are resulting in a stretched SACT delivery service sometimes resulting in delays to treatment for cancer patients. Even those infusion services who are managing to keep their service within capacity are worried about what the future demand may bring in terms of increased volume of patients requiring treatment. This could overload the infusion service in the near future and result in delays to treatment for cancer patients and a poor treatment experience.
- SACT delivery sites don’t currently have a tool that is fit for purpose in enabling them to visualise their current and future capacity and demand to plan accordingly. This along with limited resources to deliver the SACT delivery service makes focusing on optimisation difficult.
- Cancer alliances are being asked in their workforce plan to model SACT delivery service provision across all their regional SACT delivery sites.
- The NHS don’t always have the time even with access to this information and insights to address capacity issues in their SACT delivery service and plan for the future.
Project Objectives
- Visibility of current and future capacity and demand (workforce and chair capacity) within SACT delivery sites across the cancer alliance
- Optimised treatment timings and utilisation of services across at SACT delivery sites
- Improved patient experience in relation to SACT delivery across SACT delivery sites
- Improve cancer waiting times to begin SACT in line with national guidance.
- Standardisation of practices in SACT delivery
Benefits
Patient Benefits
- Reduced delay to treatment
- Improved experience of treatment due to improved capacity
- Reduction in ‘on the day’ delay and deferrals
NHS Benefits
- Increased treatment capacity to fulfil current and future demand
- Cancer Alliance has regional overview of the region’s capacity and is able to support where needed
- Reduced pressure on workforce and treatment service
MSD Benefits
- Enhanced reputation for MSD through partnership work
- Better understanding of treatment services
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of improved treatment capacity may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding & Resources
This project is a shared contribution of time between RM Partners cancer alliance and MSD.
GB-NON-09631 | June 2024
South Yorkshire & Bassetlaw Cancer Alliance MDT optimisation project for Lung, Skin, Urology and Upper gastrointestinal (UGI).
Project Title
South Yorkshire & Bassetlaw Cancer Alliance MDT optimisation project for Lung, Skin, Urology and Upper gastrointestinal (UGI).
Organisations involved
South Yorkshire & Bassetlaw Cancer Alliance
Sheffield Teaching Hospitals Foundation trust
Doncaster & Bassetlaw Foundation trust
Rotherham Foundation Trust
Barnsley Foundation Trust
Chesterfield Foundation Trust
MSD UK (Ltd)
Summary
The project will provide project management support 1.5 days per week for 12 months to support South Yorkshire and Bassetlaw Cancer Alliance to implement MDT optimisation in Lung, Skin, Urology and Upper Gastrointestinal (UGI) tumour sites in the 5 foundation trusts – Sheffield, Doncaster & Bassetlaw, Rotherham, Barnsley, and Chesterfield.
The pre-MDT standards of care will be developed by the Trusts in conjunction with the Cancer Alliance, and MSD will support in implementing the standards across the local and regional MDTs. MSD will only be involved in the implementation and not the development of the standards. The project will aim to drive forward National recommendations and guidance for MDTs to deliver an improved MDT process (1). This will be done by ensuring streamlined processes/ standards of care pathways are developed and implemented to make the best use of clinical time and resources therefore improving the efficiency of the MDT meetings.
As a result, patients may benefit from an accelerated review through the pathway, either in MDT, or outside of MDT, using pre-agreed protocols for those less urgent or severe cases. This could allow for treatment decision to be made earlier and therefore earlier access to treatment, moreover reducing patient anxiety and potentially improved access to personalised treatment options, and will also support the NHS trusts to achieve the NHSE 62 day cancer wait time target.
Background
Research has shown that by 2035, the number of patients diagnosed with cancer in the UK could reach 500,000 (1), a material increase from the 357,000 who were diagnosed in 2014.
Multidisciplinary Team meetings are seen as the ‘gold standard’ to cancer management whereby a cross sector of health professionals including of histopathologists, radiologists, surgeons, cancer nurse specialists, oncologists, and administrators/MDT coordinators with additional members dependent on the individual cancer tumour site meet to discuss individual patients’ cases and make treatment recommendations. Multi-disciplinary working continues throughout the patient pathway through diagnosis to treatment and ongoing care however the multi-disciplinary team meeting is a key discussion forum where cases are presented, a range of information shared and reviewed, and care management discussed and documented.
MDT meetings typically occur weekly and can last several hours. (2) Staff increases have not kept pace with the increase in the number of cases and so this has meant that proportionately more of clinician’s time is spent preparing for and being involved in MDT discussions with a significant amount of organisation, scheduling, clinical input and administration time/oversight processes are involved in MDT working in general and more specifically in preparing/contributing to and following up to/from the MDT-meeting. The number of patient discussions per MDT has increased with proportionately the amount of time available for each patient discussion being reduced.
Project Objectives
- An assessment of MDT meetings operating efficiencies within lung, skin, Urology, upper GI pathways across the South Yorkshire & Bassetlaw region.
- Identification of improvement initiatives to address inefficiencies linked to MDT streamlining principles and promote geographical equitable patient experience of care across all the foundation trusts.
- Provide a regional consistent approach of MDT referrals via proformas with minimum MDT referral data sets for both trust level MDTs, and regional specialist MDTs to drive quicker treatment decisions.
- Development and Implementation of localised standards of care which will be based on national or international standards, guidelines and protocols, and best practice as determined by the Cancer Alliance tumor pathway board.
Benefits
Patient
- A potential for earlier communication of a treatment plan, due to a more efficient MDT process.
- Through reduced delays, more efficient communication to patient from the MDT, and therefore the potential for less anxiety related to process.
- Improved patient outcomes.
NHS
- Improved cancer wait times to support achievement of NHS 28 -day and 62-day cancer wait time targets.
- Standardisation and optimisation of practice through sharing of knowledge and experience.
- Fiscal savings due to decrease in patient breaches.
MSD
- Greater understanding of MDT process and decision making within the NHS.
- Increased collaborative working opportunities through building trust and effecting change.
- The intended collaboration may enable more patients to have access to treatment options in line with NICE guidelines which may or may not include MSD medicines.
Funding & Resources
This project is a shared contribution of time between South Yorkshire & Bassetlaw Cancer Alliance and MSD.
GB-NON-09376 | May 2024
(1)Cancer Research UK (2017) Meeting Patients Needs – Improving the Effectiveness of Multidisciplinary Team Meetings in Cancer Services, https://www.cancerresearchuk.org/sites/default/files/full_report_meeting_patients_needs_improving_the_effectiveness_of_multidisciplinary_team_meetings_.pdf [Accessed May 2024]
(2) Independent Cancer Taskforce. (2015). Achieving World-Class Cancer Outcomes: A Strategy for England 20152020. London: Independent Cancer Taskforce. https://www.cancerresearchuk.org/sites/default/files/achieving_world-class_cancer_outcomes_-_a_strategy_for_england_2015-2020.pdf [Accessed May 2024]
Lancashire Teaching Hospital Trust Skin and Urology Diagnostic histopathology Pathway Development Programme
Project Title
Lancashire Teaching Hospital Trust Skin and Urology Diagnostic histopathology Pathway Development Programme
Organisations involved
Lancashire Teaching Hospital Trust / MSD
Summary
The intent of this collaboration is to optimise the Skin and Urology diagnostic histopathology pathway in the Lancashire Teaching Hospital Trust laboratory with the purpose of improving pathway service quality, efficiency, and productivity. This will aim to ultimately support the achievement of the Royal College of Pathology KPI of 90% of samples received have diagnostic results reported within 10 calendar days, which directly impacts the NHS 28-day Cancer Wait Time target¹. The achievement of the cancer waiting time targets has the potential to lead to improvements in patient experience of care. The desired outcome of this project is to see an improved turnaround time for histopathology diagnosis in the skin & urology diagnostic pathway at Lancashire Teaching Hospital Trust laboratory within 6 months of completion. This will be delivered through pathway mapping and gap analysis, pathway optimisation workshops and pathway improvement implementation.
Background
The Lancashire Cancer alliance is looking to identify common areas of issues within its histopathology pathways, which, if improved could positively impact the 28-day Faster Diagnosis Standard (FDS) target which is currently below the 75% target. Lancashire Teaching Hospital Trust are seen as leaders in the area of histopathology, their aim on improving the Skin and Urology diagnostic pathway could lead to improvement of the pathology target of 90% of diagnostic cases being reported within 10 days of sample receipt. This can significantly impact patients’ diagnosis and experience.
Project Objectives
- Gap analysis and pathway mapping of the Skin and Urology diagnostic histopathology pathway
- Achievement of the improved turnaround times measured against the Royal College of Pathology KPI
- Optimisation of service delivery by improving the laboratory processes
- Quicker diagnosis of Skin and Urology Tumours with an increase in number of patients receiving timely diagnosis within NHS guidance timelines.
Benefits
Benefits/ Impact to patients (if any)
- An improved patient experience of the Skin and Urology Tumour Diagnostic Pathology pathway in Lancashire Teaching Hospital Trust.
- Reduction in turnaround time 12 months post change and hence improving the time to diagnosis.
Benefits to the NHS partner
- Achievement of the improved turnaround times and measure against Royal College of Pathology KPI of 90% of samples received reported within 10 calendar days
- Earlier referral, diagnosis and treatment of Skin and Urology cancer patients
- Optimisation of service delivery
Benefits to MSD
- Better understanding of Skin and Urology tumour patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding & Resources
This project is a shared contribution of time between Lancashire Teaching Hospital Trust and MSD.
References
- https://www.thepathologycentre.org/wp-content/uploads/2018/06/CP-WEB-INS-016.UN-16.1-Turnaround-times-V1.1-1.pdf [Accessed April 2024]
GB-NON-09194 | April 2024
Immunotherapy Service Mapping in Swansea Bay and Hywel Dda University Health Boards
Project Title
Immunotherapy Service Mapping in Swansea Bay and Hywel Dda University Health Boards
Organisations involved
MSD and Swansea Bay University Health Board
Summary
Swansea Bay University Health Board (SBUHB) and Hywel Dda University Health Board (HDUHB) together as an immunotherapy service task group are working in collaboration with MSD UK to map out and baseline the current service for dealing with oncology patients that receive immunotherapy (IO).
SBUHB and HDUHB together form South West Wales cancer services with SBUHB being the hub for IO support. The two health boards wish to map out their current service with the aim of then enhancing and upskilling services across the hub and spoke service. It is anticipated that the project will be completed within 4 months.
Background
The project is needed because:
- More and more oncology patients are now on immunotherapies and by default the number of patients that require expertise from IO specialists has increased
- IO specialist advice is currently delivered by consultant level staff
- Mapping out the current service and timings associated with it will provide the health boards with the information needed to evaluate their service and compare other models from around the UK and how they can draw upon them to enhance their levels of local service
Project Objectives
- Map out the current service and patient journey associated with patients receiving immunotherapy across the hub and spoke model that exists between SBUHB and HDUHB
- Provide a clear graphical representation using Lucid Charts computer software of the service and the timelines associated with it
- Establish a baseline of the current service which (post this project) can then be compared with other similar geographical areas in the UK with the aim of enhancing and upskilling the services in Hywel Dda and Swansea Bay UHBs
Benefits
Patient Benefits
- Receiving better and more efficient care whilst receiving immunotherapy
NHS Benefits
- Potentially scalable service changes that could be rolled out across the two health boards
- Expansion of existing skill base within SBUHB and HDUHB
- Freeing up existing expertise in SBUHB and HDUHB through expansion of skills and service
MSD Benefits
- MSD reputation through working with the NHS
- Patients receiving immunotherapy will be managed more efficiently may enhance the NHS experience of IOs. This project may lead to a potential increase of treatment rates with IOs in line with NICE guidelines. As a consequence, MSD may or may not benefit from it.
Funding & Resources
This project is a shared contribution of time between Swansea Bay University Health Board and MSD.
GB-NON-08532 | November 2023
Earlier Diagnosis of Ovarian & Kidney Cancer in Brighton & Hove
Project Title
Earlier Diagnosis of Ovarian & Kidney Cancer in Brighton & Hove
Organisations involved
Sussex Integrated Care Board and MSD-UK
Summary
This project aims to address the apparent inequalities in Brighton and Hove around mortality rates for ovarian and renal cancers identified within the 2023 Cancer Joint Strategic Needs Assessment [JSNA] report & the Inequalities in mortality involving common physical health conditions England: Office for National Statistics, Aug 2023. The collaboration aims to increase early detection and diagnosis of people with ovarian and renal cancers.
MSD will work in partnership with Sussex Integrated Care Board to provide project management support to the implementation and evaluation of work to address this.
The project is planned to run for 12 months with the final evaluation 6 months post implementation of all healthcare professional educational activities.
Background
The early detection & diagnosis rate of patients with ovarian cancers is lower in Brighton & Hove vs. other Sussex localities, partially due to its often-asymptomatic presentation or symptoms that appear later that are not obvious/non-specific (often)1,2. The mortality rates for ovarian and renal cancers in Brighton & Hove are higher than in other Sussex localities3. People with symptoms can confuse ovarian and renal cancer symptoms with symptoms of “non-malignancy”, resulting in people delaying attending or being diagnosed by their General Practitioner [GP].
Symptom awareness for both cancers amongst clinicians involved in primary diagnosis needs to be heightened and current diagnostics support tools are being sub-optimally used to support the possible/probable flagging of ovarian or renal cancer which may require further specific diagnostic intervention.
The plan is to improve the recognition of the symptoms of both cancers earlier. Better use of cancer diagnostic support tools should lead to more accurate referrals, resulting in earlier diagnosis & treatment. The longer-term ambition is that this will start to address the higher levels of mortality for renal and ovarian cancers in Brighton & Hove, preventing further increases above the national average.
Project Objectives
- Reduce the current inequalities by increasing early detection & diagnosis rate of patients with ovarian and renal cancers.
- Support the provision of enhanced education and training support to increase knowledge regarding symptom awareness in undiagnosed Ovarian and Kidney Cancer including a clear definition of the issues that currently contribute to later stage diagnosis in ovarian & renal cancers to all GPs, Nurses and Pharmacists (+other Allied Healthcare Professionals [AHP’s]) who make clinical assessments of patients in primary care.
- Drive increased uptake in the use of the already integrated Ardens Pro and Ardens-EMIS urgent ‘Suspected Cancer Referral Forms’ templates for Gynaecological & Urology cancer.
- Produce a report/case study capturing the learnings that make a case for further prioritisation of ovarian & renal cancer symptom awareness and education on the best practice diagnostic steps to be adopted at scale across the Surrey & Sussex Cancer Alliance [SSCA].
Benefits
Benefits to the patient
- Recognition of the current population diagnosis inequality will be translated into improved symptom education for healthcare professionals to support earlier identification of ovarian & kidney cancer.
Benefits to the NHS
- Educational programme delivered through project management support, to help primary care HCPs in identifying symptoms of ovarian and kidney cancer.
- An understanding & a sequential plan of education activities to raise the importance & increase the clinical awareness around the diagnostic criteria for Ovarian & Renal cancer & their overlap with more common conditions as part of non-specific symptoms pathway.
- An increased cancer referral conversion rate (i.e., percentage of urgent suspected cancer referrals which result in a diagnosis of ovarian and kidney cancer)
- Development of a case study, which can be used to spread any learning and improvements in care across Sussex & nationally.
- A report of the data systems included specifically for ovarian cancer within gynaecology and renal within urology datasets.
- An audit baseline survey, at project completion (12-months from project start date) plus full evaluation 6-month post project completion of primary care Healthcare Professionals [HCPs] regarding the use of Ardens-Pro decision support tool to aid diagnosis and current detection rates for the 2 cancers.
Benefits to MSD
- Experience supporting earlier diagnosis of kidney & ovarian cancer in primary care aligned to the Long-Term Plan goal of increasing the number of patients diagnosed at stage 1 & 2.
- A better understanding of the challenges experienced along the diagnostic pathway to ensure informed conversations internally and with external stakeholders.
- A clearer understanding of the impact of clinical decision support tools to increase earlier diagnosis rates (vs national average) in an ICB setting (importance and value) in the identification of early-stage kidney& ovarian cancer.
- An enhanced reputation through partnership work and supporting the at scale replication of this approach across a system population.
- An increase in patients diagnosed at earlier stages may enable access to appropriate treatment options in line with NICE guidance, which may or may not include MSD medicines.
- Case study, which can be used to share any learning and improvements in care across Sussex & nationally.
Funding & Resources
This project is a shared contribution of time and expertise between Sussex Integrated Care Board and MSD.
GB-NON-08643 | November 2023
References:
- 1. Cancer in Brighton & Hove, 2022 – Published 6th April 2023
- 2. Ovarian cancer: the recognition and initial management of ovarian cancer – NICE CG122
- 3. Inequalities in mortality involving common physical health conditions England: 21 March 2021 to 31 January 2023 Office for National Statistics (ONS), released 31 August 2023
Clatterbridge Cancer Centre Cancers of Unknown Primary (CUP) Pan Tumour Diagnostic Pathway Development Project (PDP)
Project Title
Clatterbridge Cancer Centre Cancers of Unknown Primary (CUP) Pan Tumour Diagnostic Pathway Development Project (PDP)
Organisations involved
MSD-UK
Clatterbridge Cancer Centre
Summary
A national optimal CUP Pan Tumour Diagnostic pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all CUP patients receive optimal cancer care. There is an opportunity in Merseyside to optimise the CUP Pan Tumour diagnostic pathway in line with the national optimal CUP Pan Tumour pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved CUP Pan Tumour Diagnostic pathway aligned with the national optimal pathway and achievement of the 28-days Cancer Waiting Time Targets. The project intends to run for approximately 6 months.
Project Objectives
The primary objective of this project is the optimisation of CUP Pan Tumour Diagnostic pathways across Merseyside. Specifically contributing towards; –
- An optimised CUP Pan Tumour Diagnostic pathway aligned to the national optimal timed CUP
- Achievement of the CUP Pan Tumour Diagnostic 28 days Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the CUP Pan Tumour Diagnostic CUP Pan Tumour Diagnostic pathway in Merseyside.
- Quicker diagnosis and treatment of CUP PAN tumour and hence improving the chance of successful treatment.
NHS Benefits
An optimised pathway CUP pan tumour across Merseyside hospital sites resulting in
- Achievement of the improved turnaround times and measure against 28 days Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of CUP Pan Tumour patients
- Increase in treatment rates for CUP Pan Tumour.
- Optimisation of service delivery
MSD Benefits
- Better understanding of CUP Pan Tumour patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD
GB-NON-08682 | December 2023
MDT Reform Project- Cheshire and Merseyside Cancer Alliance
Project Title
MDT Reform Project- Cheshire and Merseyside Cancer Alliance
Organisations involved
Cheshire and Merseyside Cancer Alliance c/o The Clatterbridge Centre NHS Foundation Trust
MSD UK (Ltd)
Summary
The project will provide project management support two days per month for 8 months to support Cheshire and Merseyside Cancer Alliance to implement multi-disciplinary team (MDT) reform. The project will focus on implementing MDT triage at The Countess of Chester Hospitals (COCH), and will initially focus on breast, skin, urology and colorectal. The pre-MDT triage standards will be developed by the Trusts in conjunction with the Cancer Alliance, and MSD will support in implementing the standards across the four MDTs. MSD will only be involved in the implementation and not the development of the standards. The project will aim to drive forward National recommendations and guidance for MDTs to deliver an improved MDT process (1). This will be done through improving the effectiveness of cancer MDTs; ensuring streamlined processes/ standards of care pathways are developed and implemented to make the best use of clinical time and resources.
As a result of this project, patients may benefit from an accelerated review through the pathway, either in MDT or outside an MDT, using pre-agreed protocols for those less urgent or severe cases. As a result, treatment decisions are likely to be made earlier than in current practice, thus facilitating accelerated treatment timelines, less anxiety for patients waiting for this decision, and potentially patients deemed as fit for additional treatment options.
Background
Multidisciplinary teams (MDTs) were introduced in the late 1990s and early 2000s. Their purpose was to increase evidence-based practice and prevent implementation of treatments outside of accepted standards. MDTs are considered the gold standard for cancer patient management and mandated by the National Cancer Plan in 2000 (2); with the pledge all patients with cancer have their care reviewed by an MDT(3). However, the health services have changed significantly since their introduction and MDTs have come under increasing pressure due to:
- Significant increases in caseload
- A change in case-mix including patients with greater comorbidities because of an ageing population and increasing number of complex treatment options
- This increase in numbers/ complexity of cases to be discussed has not been matched by any increase in time set aside for the MDT
- Some MDT meetings are sometimes poorly attended by individuals, others by speciality expertise, and there are issues relating to consistent, reliable information technology, data collection and infrastructure such as videoconferencing
Cheshire and Merseyside Cancer Alliance require project management resource to support the implementation of MDT reform standards, and to implement these standards into The Countess of Chester Hospital.
Project Objectives
To provide project management support in the implementation of MDT triage process, as part of the MDT reform standards at The Countess of Chester Hospital to streamline MDT processes and improve the effectiveness of MDTs
- Implementation of Pre-MDT triage meeting
- Responsibility of the communication of outcomes to the patient should be made clear on the MDT outcome
- Consistent process to ensure that patients’ wishes are represented at MDT, to inform meeting discussions and to ensure patients are part of the planned treatment process
- Review of MDT co-ordinator training to ensure all co-ordinators have completed relevant training
- To use this as a pilot, and learnings will be shared across Cheshire and Merseyside with the aim that MDT reform will be rolled out across the region, to include all MDTs
Benefits
Patients:
- Treatment decisions in a shorter timeframe, which may give access to wider treatment options
- Potential to maintain fitness levels in a pathway with shorter duration
- Improved patient experience
NHS:
- Work more efficiently to address capacity challenge and reduce backlogs
- More time to devote to complex patients
- More appropriate patients reaching a broader treatment decision
MSD:
- Supporting our NHS collaborators in the early identification of patients suitable for treatment
- Deeper understanding of MDT processes and decision making
- This intended collaboration may enable more patients to have access to treatment options in line with NICE guidelines which may or may not include MSD medicines
Funding & Resources
This project is a shared contribution of time between Cheshire and Merseyside Cancer Alliance and MSD
GB-NON-08252 | October 2023
References:
(1) NHSE -Streamlining Multi Disciplinary Team meetings- Guidance for Cancer Alliances, 14th January 2020 NHS Gateway reference number: 000590
(2) A policy framework for commissioning cancer services: A report by the Expert Advisory Group on Cancer to the Chief Medical Officers of England and Wales (The Calman-Hine Report) April 1995
(3) The NHS Cancer plan: a plan for investment, a plan for reform. Department of Health, September
The Christie Post operative/ Adjuvant Clinic Pilot for Cancer Patients
Project Title
The Christie Post operative/ Adjuvant Clinic Pilot for Cancer Patients
Organisations involved
MSD (UK) Ltd and The Christie NHS Foundation Trust
Summary
The project will be an eighteen-month pilot at The Christie, with a proposed start date of September 2023. Currently, post-operative renal and melanoma patients are managed in a mixed clinic setting. This pilot proposes a dedicated post-operative/ adjuvant service for this patient cohort. Patient throughput in both the mixed clinic and post-operative/ adjuvant service will be measured throughout the duration of the project. The service would be nurse led, supported by a cancer nurse specialist (CNS) team and clinical expertise provided by an oncology consultant.
Background
The Christie NHS Foundation Trust is the largest cancer network in the UK, managing patients from the 3.2 million population across Greater Manchester and providing second opinions for patients nationally. The Christie historically sees 250-300 new patients per year within the renal oncology service, and 150 new patients per year within its melanoma service. With increasing numbers of new patients, and a national drive to diagnose and treat early-stage cancers, clinicians are faced with a backlog of patients and increasing capacity challenges. The Christie estimates that an additional 100-120 new renal cell carcinoma (RCC) patients and 60-70 new melanoma patients will be referred for post-operative management over the next 12 months. This represents nearly a 50% uplift in new patient activity. It will be increasingly difficult to ensure these patients are effectively managed and treated within the mixed clinic setting and there is a real need to streamline this service further.
Project Objectives
- To demonstrate the viability of a nurse led post operative/adjuvant service, for patients with renal cell carcinoma or melanoma requiring post-surgery management to achieve future funding from the NHS
- The nurse will be a full-time post, providing dedicated support, developing protocols, providing psychological support, patient information and offering adverse event management support. This will include attendance at multi-disciplinary team meetings (MDT) and offering support for patients via telephone, virtual and face-to-face appointments
- To ensure patients who are eligible for adjuvant therapy will be reviewed during their therapy and for a final safety visit prior to being referred back to the local surgical service for long-term follow-up
- A partnership resource input with MSD to fund a Band 6 nurse and the NHS to provide clinical expertise through the consultant and existing nurse team
Benefits
Patients
- Dedicated service and point of contact to manage their treatment
- A bespoke service in the post operative setting could provide patients with more tailored care
- Addressing patient concerns will continuously improve and develop the service for subsequent patients
- Patient experience data may prove sufficiently compelling to lead to wider adoption of such a service in other Trusts and thus provide equity of care across the NHS
- Patients will receive standardised, consistent education, support and point of contact
- Dedicated service may improve treatment options, adherence to treatment and experience for patients
NHS
- Patients managed within a nurse led clinic may result in consultants having more time available to deal with complex cases and thus reduce capacity challenges in the NHS
- Service evaluation will support the NHS to be able to continuously improve the service (e.g., highlighting areas of need or dissatisfaction) and adapt to the increased needs of patients
- Data from the project may be supportive and compelling to lead to wider adoption of such a service, therefore reducing variation for patients across the NHS
- Dedicated clinic to providing additional nurse time will aid with reducing post COVID backlog
MSD
- NHS Stakeholders may be willing to share experience of working with MSD to support future partnerships with the wider NHS
- Reputational benefit from partnering with The Christie
- The intended benefits of piloting an Adjuvant Clinic may mean that more patients have access to treatment options in line with NICE guidance, which may or may not include MSD medicines
Funding & Resources
This project is a shared contribution between The Christie NHS Foundation Trust and MSD.
The total project cost is £60,000
GB-NON-08022 | September 2023
Virtual SACT pre-assessment using the MySunrise App across Devon and Cornwall Hospitals
Project Title
Virtual SACT pre-assessment using the MySunrise App across Devon and Cornwall Hospitals
Organisations involved
Peninsula Cancer Alliance, Technical Health Limited and MSD Ltd
Summary
MSD intend to work in partnership with Peninsula Cancer Alliance (PCA) and Technical Health Limited, to provide project management support to implement and evaluate a virtual pre-assessment option for cancer patients waiting to undergo systemic anti-cancer therapy (SACT). The virtual clinic pre-assessment option will reside within the already established MySunrise App. Within the 12 months of planned project duration, our aims are to:
- Provide a virtual option for patients to undergo SACT pre-assessment prior to coming into hospital to receive treatment
- Release clinical capacity and healthcare professional time through providing a virtual pre-assessment option
- Provide a better patient experience from not having to travel to their hospital for a clinic appointment that can be conducted virtually
- Create an evidence base and list of benefits realised to enable wider adoption of virtual pre-assessment
- Creation of a ‘blueprint’ that will enable wider adoption of the virtual pre-assessment capability
Background
The number of patients being referred for and diagnosed with cancer is increasing due to better detection and early diagnosis programmes. Patients are also living longer with cancer meaning that the overall patient volume is increasing1. This in turn increases the demand for cancer services and puts additional pressure on service capacity.
Treatment regimens are becoming increasingly more complex, adding to workforce pressures within the SACT Day Units as more time is needed to both prepare the regimens, and to administer SACT to patients. These factors further compound the capacity challenge. The Covid pandemic has proven to be a catalyst for the wider adoption of digital and virtual engagement options for patients2. This technology can be furthered harnessed by cancer services looking for ways to release capacity (measured as HCP time and outpatient appointments (OPA)) from existing pathway processes and achieve activity with the same (or less) resource.
Project Objectives
Through a pooling of project management time, subject matter knowledge and implementation experience between Peninsula Cancer Alliance, Technical Health and MSD, the virtual pre-assessment capability will deliver the following as project outcomes:
- Each hospital within the Peninsula Cancer Alliance region will have their cancer pre-assessment pathway mapped and reviewed and then developed to include an option for patients to receive a virtual pre-assessment prior to coming into hospital for SACT therapy
- Clinical capacity will be released in terms of healthcare professional time and clinic space through providing a virtual pre-assessment option
- PREMs (patient related experience measures) will be used to survey patient experience
- A service evaluation and benefits realisation summary for the use of virtual pre-assessment in cancer patients
- A best practice/ case study example that can be used to showcase the benefit of running virtual pre-assessment clinics
- A blueprint to inform and aid the wider adoption of virtual pre-assessment by other Cancer Alliances
Benefits
Benefits to the patient
- More convenient option to complete pre-assessment, removing the need to travel to the hospital
- Improved and more informed experience of the cancer treatment pathway
- Use of virtual pre-assessment clinics may potentially mean patients start on treatment earlier
- Improved options to access treatment
Benefits to Peninsula Cancer Alliance and Trusts across the region
- Reduction in OPA clinic time spent on pre-assessment in hospitals
- Release of SACT nurse capacity as time is freed up in hospitals
- Project management support for PCA to implement virtual pre-assessment across the region
- Measurement of SACT treatment rates changing as access is improved
- Reduction in physical patient volume attending clinic, freeing up space and improving room availability
- Helping to keep vulnerable groups of patients out of hospital
Benefits to Technical Health Limited
- This project will support Technical Health to test and evaluate virtual pre-assessment across the Peninsula Region
- Gain usage data to further drive adoption of virtual pre-assessment across other Cancer Alliances
- Opportunity to work with MSD and their cross-functional teams to share expertise
Benefits to MSD
- Opportunity to work with Technical Health and PCA to share expertise
- Enhanced reputation of MSD through partnership work and opportunity to scale this approach
- This intended collaboration may enable more patients to access innovative treatments in line with NICE guidance which may or may not include MSD medicines.
Funding & Resources
This project is a shared contribution of time, expertise and funding between Peninsula Cancer Alliance, Technical Health Ltd. and MSD. The total project cost is £7,250
- https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence#heading-Zero
- https://www.openaccessgovernment.org/covid-19-and-the-digital-transformation-of-the-nhs/89606/
GB-NON-07651 | June 2023
Blackpool Teaching Hospitals NHS Foundation Trust Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology Pathway Development Project (PDP)
Project Title
Blackpool Teaching Hospitals NHS Foundation Trust Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology Pathway Development Project (PDP)
Organisations involved
MSD
Blackpool Teaching Hospitals NHS Foundation Trust
Summary
A national optimal Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all (Pan Tumour (Breast, Gynaecology, Upper GI) patients receive optimal cancer care. There is an opportunity in Blackpool to optimise the Pan Tumour (Breast, Gynaecology, Upper GI) Pathology pathway in line with the national optimal Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology pathway aligned with the national optimal pathway and achievement of the Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology pathway turnaround times reduction and 28 day Faster Diagnostic Standard Cancer Waiting Time Targets. The project intends to run for approximately 6 months
Project Objectives
The primary objective of this project is the optimisation of Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology pathways across Blackpool. Specifically contributing towards; –
- An optimised Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology pathway aligned to the national optimal timed Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology pathway
- Achievement of reducing Pan Tumour (Breast, Gynaecology, Upper GI) Pathology pathway turnaround times and 28 day Faster Diagnostic Standard Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology pathway in Lancashire.
- Quicker diagnosis and treatment of Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology performance through optimisation of the histopathology turnaround times and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway in Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology across Lancashire hospital sites resulting in
- Achievement of the reduction in Pan Tumour (Breast, Gynaecology, Upper GI) Diagnostic Pathology pathway turnaround times and 28 day Faster Diagnostic Standard Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of Pan Tumour (Breast, Gynaecology, Upper GI) patients
- Increase in treatment rates for Pan Tumour (Breast, Gynaecology, Upper GI)
- Optimisation of service delivery
MSD Benefits
- Better understanding of Pan Tumour (Breast, Gynaecology, Upper GI) patient needs
- Enhanced reputation of MSD through partnership work
As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD
GB-NON-07221 | March 2023
East Lancashire Hospitals NHS Trust Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology Pathway Development Project (PDP)
Project Title
East Lancashire Hospitals NHS Trust Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology Pathway Development Project (PDP)
Organisations involved
MSD
East Lancashire Hospitals NHS Trust
Summary
A national optimal Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all Pan Tumour (Breast, Lung, Skin, Upper GI) patients receive optimal cancer care. There is an opportunity in East Lancashire to optimise the Pan Tumour (Breast, Lung, Skin, Upper GI) Pathology pathway in line with the national optimal Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology pathway aligned with the national optimal pathway and achievement of the Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology pathway turnaround times reduction, 28 day Faster Diagnostic Standard and the 31 and 62 day Cancer Waiting Time Targets. The project intends to run for approximately 6 months
Project Objectives
The primary objective of this project is the optimisation of Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology pathways across East Lancashire. Specifically contributing towards; –
- An optimised Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology pathway aligned to the national optimal timed Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology pathway.
- Achievement of reducing Pan Tumour (Breast, Lung, Skin, Upper GI) Pathology pathway turnaround times, 28 day Faster Diagnostic Standard, 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology pathway in Lancashire.
- Quicker diagnosis and treatment of Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology performance through optimisation of the histopathology turnaround times and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway in Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology across Lancashire hospital sites resulting in
- Achievement of the reduction in Pan Tumour (Breast, Lung, Skin, Upper GI) Diagnostic Pathology pathway turnaround times, 28-day Faster Diagnostic Standard, 31-day treatment target and 62-day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of Pan Tumour (Breast, Lung, Skin, Upper GI) patients
- Increase in treatment rates for Pan Tumour (Breast, Lung, Skin, Upper GI)
- Optimisation of service delivery
MSD Benefits
- Better understanding of Pan Tumour (Breast, Lung, Skin, Upper GI) patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD
GB-NON-07056 | February 2023
Immuno-oncology Nurse Led Service
Project Title
Immuno-oncology Nurse Led Service
Organisations involved
University College Hospital London (UCLH) and MSD
Summary
The collaboration will support the set up and development of an “Immuno-oncology nurse led service” for patients receiving immunotherapy treatments. This would involve UCLH recruiting a nurse to deliver and evaluate the nurse-led service for the duration of 15 months. This would be supported by a Specialist Cancer Pharmacist and Oncology Consultant at UCLH, project management support from MSD and financial input from both parties.
The project will aim to demonstrate the value for patients who are treated with immunotherapy for cancer, primarily checkpoint inhibitors, in reducing the variation of education and support across different cancer types. This will be achieved through consistent education of staff involved in managing patients on treatment and support within a dedicated immuno-oncology service.
The goal is for long term funding to be achieved after the project and to collate detailed information on the setup and running of the service that will enable other trusts nationwide to implement a similar model of care.
Background
UCLH had recently conducted a clinical audit of 62 patients that received checkpoint inhibitors (CPI) and identified a variation in the education and management of patients receiving such treatments. The current use of CPI was 123 patients per month across 5 different cancer types and they expect this to increase with both indications and new tumour groups and there is a need for patients to be educated by a trained individual in immunotherapy. This education needs to be individualised for patients that are diverse in their cancer type, intent of treatment and their prior treatment history. This will reduce the variation in support that patients receive whilst on checkpoint inhibitors. This project will aim to drive consistency of support across different cancers for patients treated with immune-oncology agents.
Project Approach
To successfully initiate an immuno-oncology service (primarily for checkpoint inhibitors), and to share the learns with peers and the wider NHS. The nurse once recruited will be involved in:
- Management of the Immuno-oncology Service, including participation in clinic, meetings and follow up.
- Supporting the patient’s journey when on immune-oncology treatment; specifically check-point inhibitors.
- Educating patients on their treatment, developing and providing patient information for a diverse population.
- Offering Adverse Event (AE) management support including advising treating clinicians on an individualised basis.
- Education of staff – such as nurses, pharmacists and oncologists on specific patient needs.
- Co-ordination of care, supporting the patients, managing investigations and appointments.
- Support innovation and improving services by adopting best practices.
- Ensuring adherence to best practice and local guidelines.
Project Objectives
The service will aim to achieve the following:
- Establishment of a dedicated service to run for 12 Months within the scope of the project, followed by 3 months evaluation.
- All new patients will have access to the educational element of the service including those that are changing therapy to an immunotherapy.
- Patients experiencing toxicities on current immunotherapy will be flagged to the service and advice given will be tailored to meet the patient’s needs and circumstances. Advice can be given either to the patient directly or via the managing clinician. The aim is that all eligible immunotherapy patients believe they have been adequately educated and given the correct information to be able to identify and report AEs in a timely manner.
- All staff working with patients being treated with immunotherapy feel adequately upskilled to support patients through their journey, measured via questionnaire across nurses, pharmacists and oncologists.
- The impact on bed days, compliance with CPI’s and the treatment rate of CPI’s will be evaluated.
- Patient experience and psychological impact will be measured.
- Funding to be secured by UCLH to continue the service after the project.
- The project will be evaluated, and the results will be published (to be decided on publications).
Benefits
Benefit to Patients
- Patient will have a dedicated service and confidence of their point of contact to manage their treatment and concerns
- Patients will receive consistent education on immunotherapy irrespective of what their cancer type alongside their tumour related education
- Patients will seek appropriate help and advice in a timely manner
- Dedicated service may improve treatment compliance
Benefits to the NHS Partner
- Patients managed within the nurse led service may result in consultants having more available time for complex cases
- There may be a reduction reduction in bed days associated with I/O usage due to the consistent education and support given
- Provide other hospitals with information to support business cases in adopting a similar service
- Addressing patient concerns will continually improve the service for subsequent patients
- Data from the project may be supportive, informative and compelling to lead to wider adoption of such a service therefore reducing variation for patients across the NHS
Benefits to MSD
- NHS Stakeholders may be willing to share experience of working with MSD to support future collaboration to the wider NHS
- Reputational benefit of working with UCLH
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of this project could be that MSD may see appropriate use of their NICE approved medicines.
Funding
This project is a shared contribution between UCLH and MSD UK Ltd. The total project cost is £70,000
GB-NON-06876 | January 2023
South Yorkshire & Bassetlaw Cancer Alliance Pan Tumour Pathology Pathway Development Project (PDP)
Project Title
South Yorkshire & Bassetlaw Cancer Alliance Pan Tumour Pathology Pathway Development Project (PDP)
Organisations involved
MSD
Sheffield Teaching Hospital NHS Foundation Trust, Barnsley Hospital Foundation Trust, The Rotherham NHS Foundation Trust, Doncaster and Bassetlaw Teaching Hospital Foundation Trust and Chesterfield Royal Hospital Foundation Trust
Summary
There is an opportunity in the South Yorkshire &Bassetlaw and Chesterfield area to optimise the pan tumour pathology testing pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved pan tumour pathology pathway and achievement of the relevant targets associated with, head and neck/upper GI/skin/breast/lung/gynaecological cancers, 28 day Faster Diagnostic Standard and the 31 and 62 day Cancer Waiting Time Targets. The project intends to begin on 1st October 2022 and anticipates a finish date on 30th September 2023
Project Objectives
The primary objective of this project is the optimisation of pan tumour pathologypathways across South Yorkshire & Bassetlaw and Chesterfield. Specifically contributing towards; –
- An optimised pan tumour pathology pathway
- Achievement of the head and neck/upper GI/skin/breast/lung/gynaecological cancers 28 day Faster Diagnostic Standard, 31-day treatment target and 62 day referral to treatment) Cancer Waiting Time targets
Project Approach
- Pathway mapping of each pathology service in South Yorkshire & Bassetlaw and Chesterfield and creation of Lucid charts depicting the current pathway
- Gap analysis contributing towards co-creation of service re-design plans from gap analysis outputs for each site managing head and neck/upper GI/skin/breast/lung/gynaecological cancers patients in South Yorkshire & Bassetlaw and Chesterfield
- Implementation of an optimised pathway for each site managing head and neck/upper GI/skin/breast/lung/gynaecological cancers patients across South Yorkshire & Bassetlaw and Chesterfield
- Both parties commit to measuring the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the head and neck/upper GI/skin/breast/lung/gynaecological cancers pathway in South Yorkshire & Bassetlaw and Chesterfield.
- Quicker diagnosis and treatment of head and neck/upper GI/skin/breast/lung/gynaecological cancers and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway in head and neck/upper GI/skin/breast/lung/gynaecological cancers across South Yorkshire & Bassetlaw and Chesterfield hospital sites resulting in
- Achievement of the head and neck/upper GI/skin/breast/lung/gynaecological cancers 28 day Faster Diagnostic Standard, 31-day treatment target and 62 day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of head and neck/upper GI/skin/breast/lung/gynaecological cancers patients
- Increase in treatment rates for head and neck/upper GI/skin/breast/lung/gynaecological cancers
- Optimisation of service delivery
MSD Benefits
- Better understanding of head and neck/upper GI/skin/breast/lung/gynaecological cancers patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
Total Project = £36,278.87; MSD contribution = £19,900; NHS Contribution = £ 16,378.87
GB-NON-06488 | September 2022
Pan Tumour - Completed Projects
Greater Manchester (GM) Cancer Alliance – Multi-disciplinary team (MDT) Reform Implementation project
Project Title
Greater Manchester (GM) Cancer Alliance – Multi-disciplinary team (MDT) Reform Implementation project
Organisations involved:
GM Cancer Alliance
MSD UK Ltd
Summary
The aim of the project was to provide project management support for 6 months to support the implementation of the Greater Manchester (GM) Cancer Multi-Disciplinary Team (MDT) standards within NCA (Northern Care Alliance), focusing on usage of the patient Impact Statement and to implement these standards into the annual quality assurance process. The standards were developed by GM Cancer Alliance, as part of an eighteen-month MDT Reform project.
Additionally, MSD provided funding for an MDT toolkit to be utilised by MDTs to share best practice and advice on how best to achieve the GM MDT standards, and the toolkit was rolled out as part of the project.
At the start of the project an initial baseline was taken of usage of the Patient Impact Statement by MDTs within NCA, with the majority of MDTs stating that this was already being done, although there was no formal or consistent process in place. It was decided that project management support would then be dedicated to one MDT- Salford Royal colorectal team. MSD supported with development of an action plan for MDT reform, and the GM team would then implement the agreed MDT reform standards and utilisation of the Patient Impact statement. The aim was to also develop consistent processes that could be audited and replicated.
Benefits Realised
By providing project management support, MSD has supported GM Cancer to create an MDT reform toolkit, and to implement MDT reform standards at Salford Royal colorectal lower GI MDT. The project supported with implementation of the following standards:
- MDT reform toolkit to support implementation of MDT reform and standards across Greater Manchester and wider NHS
- Utilisation of patient impact statement (piloted in patients through fast-track endoscopy)
- A standardised referral proforma has been developed with mandatory fields regarding performance status, frailty and any other issues which may effect MDT decision- thus improving quality of information to appropriately discuss patients at MDT. Which has proceeded to be live on the electronic referral system.
- Pre MDT triage meetings
- Communication of outcomes to patients via telephone (CNS led) within 2 days of MDT
Patient benefits:
- Utilisation of the patient impact statement ensures the patient voice is heard at MDT, to ensure their wishes are part of the MDT decision making process and allowing patients to decide how they would receive their MDT decision.
- June -December 2023 40 patients completed patient impact statement. 23 patients requested a telephone appointment in nurse led clinic directly after MDT, 10 patients requested a face-to-face consultant appointment, 5 requested any method felt suitable.
- The patient impact statement is live on the electronic referral system. This was initially trialled in patients through the endoscopy department and has now been extended across MDTs within Greater Manchester
Patients receiving MDT decision via the CNS telephone service have been selected from the patient impact statement or at MDT. This new service has resulted in patients receiving faster outcome of MDT decision, with the clinic taking place directly after MDT. The clinic has dedicated admin support and a tariff attached, with letters being sent to the patient and GP within 48 hours of MDT. May 2023 to 4th January 2024- 106 patients have received telephone appointments in the nurse led clinic.
NHS benefits:
- MDT reform tool-kit has supported clinicians across GM and wider NHS with resources on MDT reform. There have been 314 views of the main GM Cancer MDT Reform webpage, 146 collective downloads of the MDT Reform Standards and MDT reform Toolkit between18 Sept 2023 – 18 Jan 2024.
- Standardising referral to improve quality of information to meet the minimum data set required for effective decision making and care planning
- Pre MDT triage meetings led by CNS team and supported by colorectal consultant uses an agreed algorithm to remove patients or delay discussions. This has improved efficiency of MDT and time spent of patients needing discussion.
- The CNS led telephone service post MDT reduces the number of face-to-face appointments and has resulted in patients receiving MDTs results sooner (aim is within 48 hours of MDT).
- NCA adding MDT reform standards to the annual quality assurance process to ensure consistency across MDTs within NCA.
- Greater Manchester Cancer Alliance have now committed to a full time project manager to continue MDT reform implementation within Greater Manchester.
MSD benefits:
- Greater understanding of MDT processes and challenges to implement MDT reform.
- Enhanced reputation of MSD through partnership working.
- This collaboration may have enabled more patients to access innovative treatments in line with NICE guidance. which may or may not have included MSD medicines.
Funding & Resources
This project was a shared funding commitment from Greater Manchester Cancer & MSD.
Lessons learnt
- MDT reform is complex and a lengthy process, involving multiple stakeholders. A short duration project should focus on specific parts of the MDT process. Greater Manchester Cancer Alliance will now be continuing with MDT reform and have recruited a dedicated project manager to lead the project, with ongoing evaluation of the effectiveness of the changes.
- IT changes to support MDT reform are difficult as often different systems are involved and any planned changes should be addressed at the start of the project to allow for time delays.
- Tight project planning and a clear communication plan, with an NHS champion to ensure all stakeholders are aware of the project and to support with buy in to the project.
Publications
At the time of writing there are no plans for the NHS to publish this data.
GB-NON-08947 | April 2024
Oxford University Hospitals NHS Foundation Trust Cancer Prescription Screening and Treatment Pathway Development Programme
Project Title
Oxford University Hospitals NHS Foundation Trust Cancer Prescription Screening and Treatment Pathway Development Programme.
Organisations involved
Oxford University Hospitals NHS foundation trust and MSD.
Summary
There is an opportunity in Oxfordshire to optimise the cancer treatment and prescription screening pathway to improve the service quality, service efficiency, productivity, and patient experience. The desired outcome of this project is an improved treatment pathway and achievement of the 31 and 62 day Cancer Waiting Time Targets.
Benefits Realised
Due to significant and unanticipated time capacity challenges within the Oxford system which needed to take priority, the full benefits of this prescription screening project were not fully realised as the project was not completed.
Funding & Resources
This was a project with shared time commitment from Oxford University Hospital NHS FT & MSD
Lessons learnt
Creation of a formal contingency plan should any of the key stakeholders have time capacity issues, leave their post or their organisation during the implementation of the project.
Publications
No publications are planned.
GB-NON-08941 | February 2024
University Hospitals Plymouth Chemo capacity and treatment delivery Pathway Development Project (PDP)
Project Title
University Hospitals Plymouth Chemo capacity and treatment delivery Pathway Development Project (PDP)
Organisations involved
University Hospitals Plymouth NHS Trust and MSD
Summary
There is an opportunity in Plymouth, Devon to optimise the Cancer treatment and prescription pathway to improve the service quality, service efficiency, productivity, and patient experience. The desired outcome of this project is an improved Treatment pathway and achievement of the 31- and 62-day Cancer Waiting Time Targets. The primary objective of this project is the optimisation of treatment and prescription pathways across Plymouth, Devon.
Benefits Realised
Due to significant and unanticipated capacity issues within the Plymouth system which needed to take priority, the Trust made the decision to cancel the Chemo capacity and treatment delivery planned pathway development workshops, the mainstay of the project plan.
Funding & Resources
This was a project with shared time commitment from University Hospitals Plymouth & MSD
Lessons learnt
The importance of a strong sequential stakeholder engagement & communication plan to support time management. The need to gain agreement from service management to clinical & administrative stakeholders for protected time to prioritise improvement project.
Publications
No publications are planned.
GB-NON-08940 | February 2024
Somerset Wiltshire Avon and Gloucestershire (SWAG) Cancer Alliance Prehabilitation Patient Information Platform
Project Title
Somerset Wiltshire Avon and Gloucestershire (SWAG) Cancer Alliance Prehabilitation Patient Information Platform
Organisations involved
SWAG Cancer Alliance and MSD
Summary
The goal of this project was to provide project management support to engage and facilitate stakeholder healthcare professionals (HCPs) around regional prehabilitation services and their input into developing a SWAG Regional Cancer Prehabilitation Information Platform. Also, to further engage regional HCP stakeholders to drive adoption and usage of the platform across their respective Trusts within the region. In doing so we aimed to:
- Reduce the variation in the quality of prehabilitation services across the region
- Provide project management support to facilitate regional standardisation of prehabilitation services
- Involve healthcare professional leads and charities to be involved in the building of the platform, ensuring that it was fit for purpose
- Increase patient engagement and education, enabling people to make more informed choices regarding their health
- Improve patient’s abilities to build mental and physical resilience’s during and beyond their cancer journey
Benefits Realised
-
Patient Benefits
- As a result of the new digital prehabilitation platform, patients and carers within the region have benefitted from the ability to access prehabilitation information, instruction, and guidance, enabling them to best prepare mentally and physically for their treatment journey
-
NHS Benefits
- The creation of a digital prehabilitation platform built using feedback, insights, and recommendations from a multi-disciplinary team of HCPs representing each of the Trusts from across the region
- Increasing access to and viewing of the platform as measured through the number of views on a weekly and monthly basis (see appendix)*. From August 23 to December 23:
- Monthly views increased from 140 to 222
- Monthly users increased from 77 to 138
- Provision of a prehabilitation resource for healthcare professionals to access, irrespective of location or position, reducing variation in access to resources across the region
- Identification of prehabilitation as a regional priority that is now listed as such on the SWAG Cancer Alliance Webpage
- Dissemination of the Prehab Hub as a digital resource to a wide range of NHS stakeholder organisations, institutes, networks and charities through presentations and written communications both regionally and nationally, including:
- A case study written for the National Living With and Beyond Cancer team who have published the case study on their NHS Futures site, and have also circulated the case study in their December 2023 newsletter
- A case study blog written for the Cancer Care Map team and posted on their web page
- Poster containing prehab digital platform details to be circulated to relevant HCPs in the region
-
MSD Benefits
- Contributing towards a new regional digital capability that has the potential to positively impact up to 14,688 patients annually**, although it’s acknowledged that not every single patient diagnosed with cancer will access the platform
- This collaboration may have enabled more patients to access innovative treatments in line with NICE guidance which may or may not have included MSD medicines.
- Enhanced reputation of MSD through partnership work
References
* – Google Analytics
** – UK cancer incidence figures – https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/all-cancers-combined#heading-Two. SWAG Cancer Alliance regional population estimate – https://www.swagcanceralliance.nhs.uk/about-us/
Funding & Resources
This was a project with shared time commitment from SWAG Cancer Alliance and MSD
Lessons learnt
A variation agreement was issued and signed to extend the running time of the project
A project lead deputy could be assigned for continuation should unforeseen circumstances disrupt the core project team
Publications
None
GB-NON-08801 | January 2024
Appendix
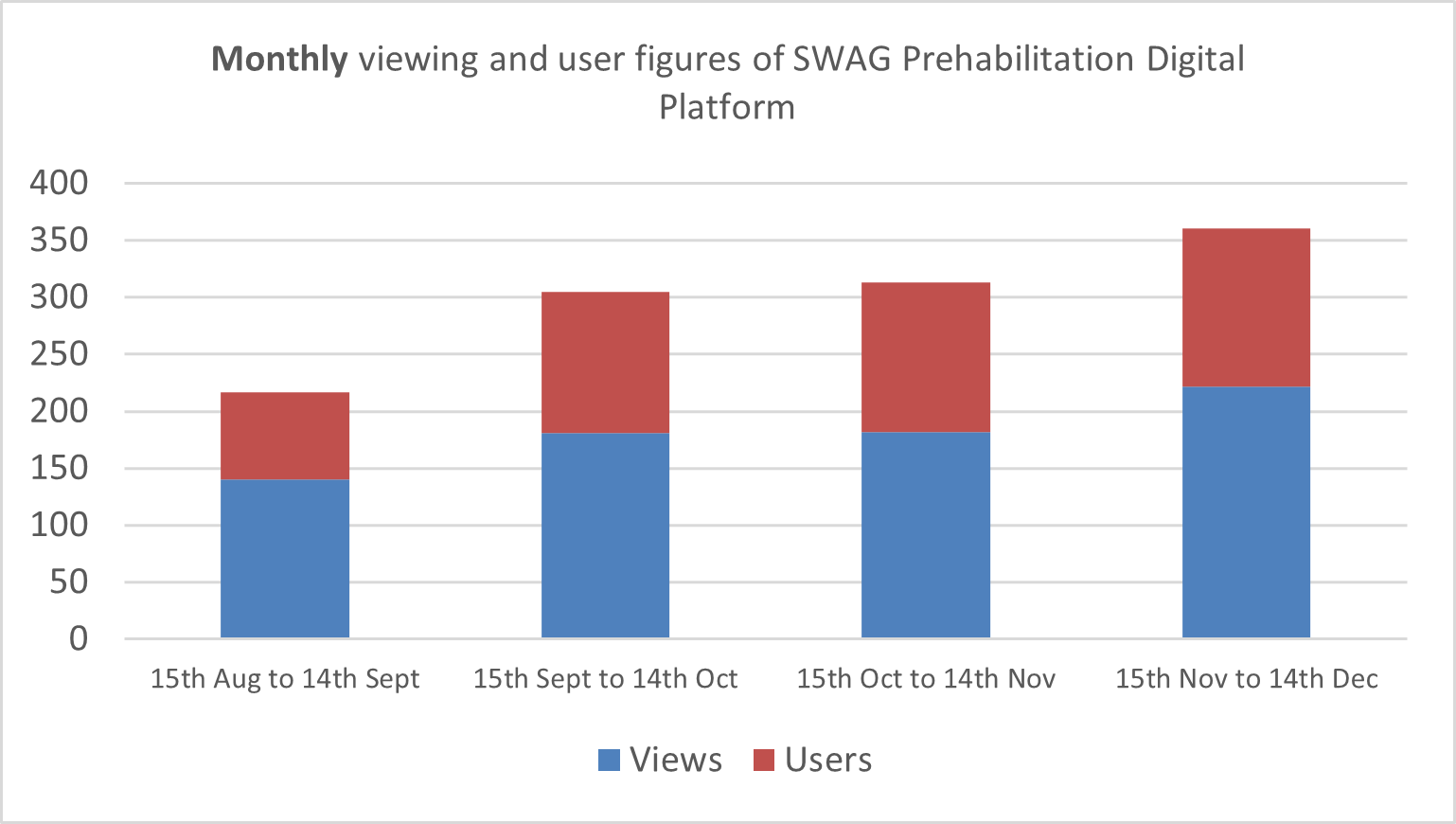
Data from Google Analytics for the SWAG Cancer Alliance Webpage. Access granted by SWAG Cancer Alliance
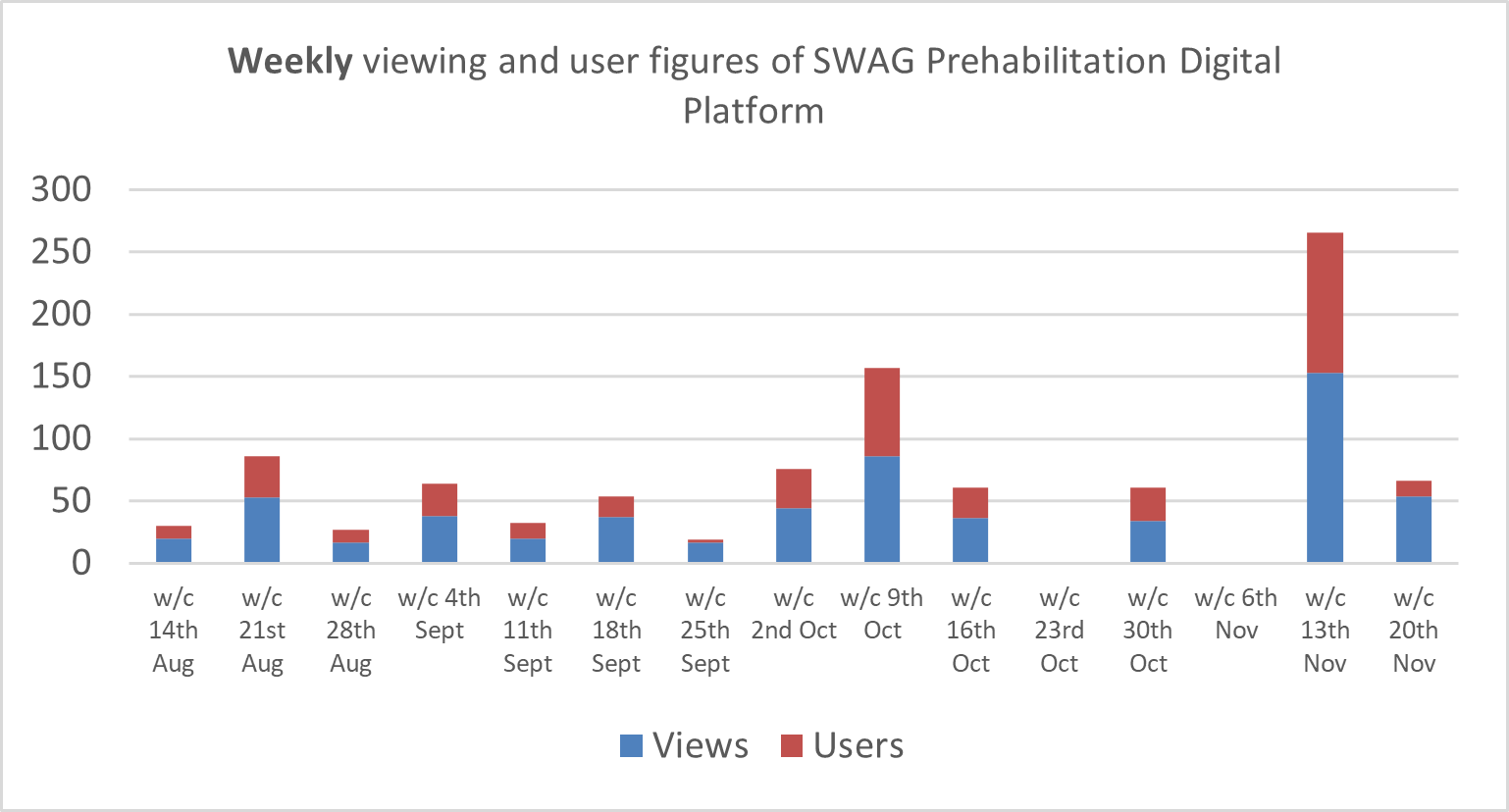
Data from Google Analytics for the SWAG Cancer Alliance Webpage. Access granted by SWAG Cancer Alliance
Skin - Active Projects
University Hospitals Sussex Foundation Trust Pathway Development Project (PDP)
Project Title
University Hospitals Sussex Foundation Trust Pathway Development Project (PDP)
Organisations involved
MSD
University Hospitals Sussex Foundation Trust
Summary
A national optimal skin cancer pathway has been created with the intention of improving patient experience through promoting quality cancer care and ensuring all skin cancer patients receive optimal cancer care. There is an opportunity in Brighton and surrounding Sussex area to optimise the melanoma pathway in line with the national optimal skin cancer pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved melanoma pathway aligned with the national optimal pathway and achievement of the skin cancer type here 28 day Faster Diagnostic Standard and the 31 and 62 day. Cancer Waiting Time Targets. The project intends to run for approximately 12 months.
Project Objectives
The primary objective of this project is the optimisation of melanoma pathways across Brighton and surrounding Sussex area. Specifically contributing towards; –
- An optimised melanoma pathway aligned to the national optimal timed skin cancer pathway
- Achievement of the skin cancer 28 day Faster Diagnostic Standard, 31-day treatment target and 62 day referral to treatment Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the melanoma pathway in Brighton and surrounding Sussex area.
- Quicker diagnosis and treatment of melanoma and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway in melanoma across University Hospitals Sussex hospital sites resulting in
- Achievement of the skin cancer 28 day Faster Diagnostic Standard, 31-day treatment target and 62 day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of melanoma patients
- Increase in treatment rates for skin cancer
- Optimisation of service delivery
MSD Benefits
- Better understanding of melanoma patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD
GB-NON-09628 | June 2024
Writing of a business case for development of skin cancer services in Thames Valley
Project Title
Writing of a business case for development of skin cancer services in Thames Valley
Organisations involved
Oxford University Hospitals NHS Foundation Trust and MSD
Summary
Provision of project management support will be given to write a business case that will be used to apply for funding for a Band 8 Nurse specialist who will work to ensure seamless and coordinated care for patients undergoing adjuvant therapy and / or surveillance monitoring within the skin cancer pathway at Oxford University Hospitals and all peripheral referring hospital sites. This is planned to take 6 months in which the business case will be written, validated, and submitted to secure permanent funding to employ a new Skin Cancer Nurse Registrar (Band 8a)
Background
The skin cancer service at Oxford and surrounding hospitals needs to be developed to address the increasing patient volumes, the introduction of adjuvant treatments approved by NICE, and the challenges in retaining staff. Currently, there are additional travel requirements for patients referred from peripheral hospitals to Oxford which may discourage them from accepting adjuvant therapy. The current referral and communication processes between peripheral hospitals and Oxford exhibit variability and inconsistency leading to variation in treatment monitoring and ongoing surveillance.
Project Objectives
- The primary objective of this project is to write a business case that will be used to apply for and secure funding for a band 8 nurse specialist who will work to ensure seamless and coordinated care for patients undergoing adjuvant therapy within the skin cancer pathway at Oxford University Hospitals and peripheral referring hospital sites.
- The business case will include changes in skin cancer patient demand observed over the last 5 years and make a case for existing workforce having to manage ever increasing demand without associated capacity increasing
- The business case will describe how a band 8 skin cancer nurse specialist will work across Oxford and peripheral referring hospitals to ensure seamless coordinated treatment and care for patients undergoing adjuvant therapy
Benefits
Benefits to the patient
- Patient care will be enhanced with increased numbers of patients potentially receiving treatment, including adjuvant treatment (vs baseline)
- Potentially start on treatment earlier with reduced waiting times, and may stay on treatment longer
- Access to clinic appointments, imaging, and surveillance in a timely manner
Benefits to Oxford University Hospitals NHS Foundation Trust
- Provision of funding for a band 8 skin cancer nurse specialist
- Appointment of nurse will lead to service capacity being optimised meaning patients will receive treatment quicker at a location closer to home
- Improvements in variation of care for their patients
Benefits to MSD
- A better understanding of NHS business case writing requirements and challenges to skin cancer pathways from increasing patient demand
- Enhanced reputation of MSD through partnership work
- Potentially improved access to innovative treatments in line with NICE guidance which may, or may not include MSD medicines
Funding & Resources
This project is a shared contribution of time between Oxford University Hospitals NHS Foundation Trust and MSD
GB-NON-09531 | May 2024
Maidstone & Tunbridge Wells NHS Trust Skin Cancer Pathway Development Project (PDP)
Project Title
Maidstone & Tunbridge Wells NHS Trust Skin Cancer Pathway Development Project (PDP)
Organisations involved
MSD-UK
Maidstone & Tunbridge Wells NHS Trust
Summary
There is an opportunity in Maidstone & Tunbridge Wells to optimise the skin cancer pathway to improve the service quality, service efficiency, productivity and patient experience. The desired outcome of this project is an improved skin cancer pathway and achievement of the skin cancer 28 day Faster Diagnostic Standard and the 31 and 62 day Cancer Waiting Time Targets. The project intends to run for approximately 12 months
Project Objectives
The primary objective of this project is the optimisation of skin cancer pathways across Maidstone & Tunbridge Wells. Specifically contributing towards; –
- Achievement of the skin cancer 28 day Faster Diagnostic Standard, 31-day treatment target and 62 day referral to treatment Cancer Waiting Time targets
- Measurement of the outcomes of the project and disseminating these outcomes within 6 months of the project completion
Benefits
Patient Benefits
- An improved patient experience of the skin cancer pathway in Maidstone & Tunbridge Wells.
- Quicker diagnosis and treatment of skin cancer and hence improving the chance of successful treatment
NHS Benefits
An optimised pathway in skin cancer across Maidstone & Tunbridge Wells hospital sites resulting in
- Achievement of the skin cancer 28 day Faster Diagnostic Standard, 31-day treatment target and 62 day referral to treatment Cancer Waiting Time targets
- Earlier referral, diagnosis and treatment of skin cancer patients
- Increase in treatment rates for skin cancer
- Optimisation of service delivery
MSD Benefits
- Better understanding of skin cancer patient needs
- Enhanced reputation of MSD through partnership work
- As a pharmaceutical manufacturer of oncology medicines, an indirect result of an improved pathway may be that MSD see more appropriate usage of their NICE/SMC approved medicines
Funding
No funding is associated with this project. The project is a shared contribution of time from both the NHS organisation and MSD-UK
GB-NON-07644 | June 2023
Other Collaborative Working
Completed Projects
SIMP-L Social Isolation Management Programme - Lothian
Project Title
SIMP-L
Social Isolation Management Programme – Lothian
Organisations involved
NHS Lothian and MSD
Summary
NHS Lothian are working with MSD to develop and support two community link worker roles focused on working with the socially isolated elderly in the south west of Edinburgh with the primary aims of improving patients’ quality of life and improving primary care capacity. In light of the Covid-19 pandemic the Community Link Workers will use their knowledge and experience to act as virtual liaison between the socially isolated elderly and the health, social care and third sector.
Background
Social isolation has detrimental effects on health, having been identified as a risk factor for all-cause morbidity and mortality with outcomes comparable to smoking, obesity and high blood pressure.
The socially isolated elderly are especially at risk with significant increases in the rates of CVD and diabetes, poorer cancer survival rates and decreased resistance to infection.
The socially isolated elderly are also the significant users of GP and practice nurse appointments, with a Royal College of General Practitioners survey finding that 75% of GPs see between one and five people a day who have come in mainly because they are lonely.1
The Scottish Government has recognised the impact on health and service capacity that social isolation can cause and recently implemented a national consultation in this area and there have been several studies looking at ways in which to tackle this issue.
In studies link worker models have been shown to have significant benefits to both patients and primary care capacity as well as positive financial outcomes for the local health economy.
Project Objectives
The project proposal involves working with NHS Lothian and the Edinburgh Health and Social Care Partnership (EHSCP) to develop and support two community link worker roles focused on working with the socially isolated elderly in the south west of Edinburgh.
The project is a pilot run for 27 months now that an evaluation of the original 15 month project signalled positive outcomes for patients and the NHS in Scotland. If successful, the model will be evaluated and may well be of interest to other health economies across Scotland.
The Link Workers would focus on socially isolated over 65’s (over 55’s in steering group approved practices) with objectives of:
- Working to prevent social isolation in at risk patients
- Improving the health/quality of life of the socially isolated
- Building community capacity so that there is a robust infrastructure to support the socially isolated
- Upskilling primary care healthcare teams to help identify socially isolated patients and signpost those patients to the appropriate support
- Reduce workload for GPs/PNs so that they can focus on healthcare management rather than dealing with social issues
Benefits
Benefit to Patients
- Aim to be less socially isolated/lonely
- Possible improved health/quality of life
- Possible prevention of illness
- Aim for an improved management of chronic illnesses
- Aim for an improved service provision allowing patients to access healthcare more easily
Benefits to NHS Lothian
The project will aim to help to meet a number of the priorities from the Edinburgh Health and Social Care Strategic Plan 2016 – 2022 including:
- Tackling inequalities
- Prevention and early intervention in disease management
- Ensuring a sustainable model of primary care
- Improving care and support for frail older people
- Improving outcomes for people living with long-term and multiple conditions
- Improving the understanding of the strengths and needs of the local population
- Living within our means
As well as potentially:
- Improving GP/PN capacity
- Reducing A&E admissions/delayed discharges
- There may be a reduction in drug spend (antidepressants, pain medications) although may not be directly attributed to the project
- Improving community capacity
- Improving service provision over the South West Locality
Benefits to MSD
- Support and reinforce MSD’s aim to be the healthcare company of choice in Scotland
- Indirectly benefit MSD as a result of potential improvements in capacity and service design, meaning more patients may be identified and they may go on to be prescribed an MSD product where deemed clinically appropriate
- Opportunity to engage as a partner with NHS Lothian rather than just a supplier of medicines
- Reputational benefits would be immediate and long lasting and the financial benefits would start to develop as more patients had access to primary care services
Funding
The project is being funded with equal allocation of resources to the project from NHS Lothian and MSD.
References
- RCGP calls for social prescriber in every practice to tackle ‘epidemic of loneliness’ http://www.pulsetoday.co.uk/news/commissioning/commissioning-topics/prescribing/rcgpcalls- for-social-prescriber-in-every-practice-to-tackle-epidemic-ofloneliness/ 20036746.article Last accessed [December 19, 2018]
GB-NON-06279 | August 2022
Over 65s Vaccination GP Cluster Project
Project Title
Over 65s Vaccination GP Cluster Project
Organisations involved
MSD and Afan GP Cluster, Swansea Bay University Health Board
Summary
The objective of the project is to increase access to the National Immunisation Programmes (NIP) applicable to the over 65 age cohort. This will be achieved through collaborating with General Practices in the Afan Cluster to increase the uptake of Flu, Shingles and Pneumococcal vaccinations in line with the respective NIP within eligible cohorts of patients across the cluster.
Background
Currently vaccination rates for shingles, pneumococcal disease and also flu are low across Wales. The creation of GP clusters across Wales was designed to allow GP surgeries to work together to achieve shared targets across patient care. Due to the resourcing issues and stretched services GP clusters have not been able to provide this cluster approach and demonstrate their potential within vaccination services.
Project Approach
This project aims to pilot the use of GP clusters to enhance vaccination services and patient uptake of these services. It is proposed that the increase in vaccination uptake will provide enough revenue through vaccination administration payments to allow this project approach to become sustainable and replicable in other geographical areas.
A partnership between MSD and the Afan cluster will enable collaboration around improving care, eligible patients will receive an increased access to vaccinations in line with Public Health Wales and the National Immunisation Programme (NIP). A remote triaging service and subsequent vaccine administration will be provided by one of the surgeries situated within the cluster (Rosedale Medical Practice) who operate some other cluster services under the name – GP Hub. This will be a 6 month service addressing 3 areas of vaccinations in the over 65 year olds. The project would focus on over 65s who are eligible to receive all 3 vaccinations thereby maximising the impact that the approach will have.
Project Objectives
The objective of this project is to increase vaccination rates amongst eligible patients for the three National Immunisation Programmes within scope.
Benefits
Patients
- An increased number of patients will receive access to vaccinations in line with Public Health Wales and the NIP
- Reduce the risk of associated complications of shingles, pneumonia and Flu
NHS
- The benefits to the GP practices will be that the respective uptake of vaccinations within their service will increase
- Reducing the incidence of associated disease within the NIP and the cost utilization to the NHS
- Increasing focus on over-burdened services such as long term conditions clinics as resource will not be pulling from existing services e.g. Diabetes- COPD
- Sustainability post MSD Exit from project – business plan with economic service benefits to take to Public Health Wales
MSD
- As a vaccines manufacturer MSD may see an increase in the uptake of certain vaccinations that they produce in line with Public Health Wales policy and the NIP
- There is an additional reputational benefit to be gained through working in collaboration with the NHS to improve outcomes for patients
Funding
The total project budget is £36,702.40. This is made up from a contribution of £21,550 by MSD and a contribution of £15,152.4 by the Afan cluster group (Swansea Bay University Health Board).
Date of Preparation: January 2022, Extended June 2025| GB-PNX-00164
Cardiff and Vale University Health Board Diabetes Informatics Programme
Project Title
Cardiff and Vale University Health Board Diabetes Informatics Programme
Organisations involved
MSD and Cardiff and Vale University Health Board
Summary
Cardiff and Vale University Health Board will work with MSD to improve the quality of their diabetes service by understanding the community’s needs better, working with the community to meet care needs earlier and by looking at new and innovative ways to help them address the challenges set out by the burden of diabetes and to standardise the approach across the practices involved.
Background
General Practice and secondary care diabetes services within Cardiff and Vale University Health Board are working collaboratively to provide coordinated, high quality clinical care, in the community, offers a positive patient experience and improves patient outcomes.
Across Wales and Cardiff and Vale UHB, there is a variation in the percentage of people with diabetes receiving the 8 care processes and achieving the NICE 3 treatment targets.
Project Approach
- Diabetes Dashboard will provide all 62 practices with their baseline diabetes performance against project deliverables and will update to reflect changes throughout the year.
- All 62 Practices will review the Diabetes Dashboard with their community diabetologist or DSN in an ongoing basis to discuss progress against the specific deliverables.
- MSD will deploy a suite of searches written by a third -party search writing company (VIPC) across all 62 Practices in Cardiff and Vale UHB. VIPC is an information management and technology solution company that specialise in search writing for General Practice. VIPC will write a suite of searches for the two IT systems across Cardiff and Vale UHB; EMIS and VISION. The specific searches will be constructed in collaboration between clinical leads within Cardiff and Vale UHB and ViPC to ensure an effective transition from the Evidence into Practice suite of searches to the VIPC suite of searches.
- The list of searches will appear in a format that visually displays the number of patients the search pertains to and the % of patients that may/may not benefit from a review.
- All 62 Practices will download the VIPC suite of searches application independently following communication from lead clinicians within Cardiff and Vale UHB. The searches will be clinically validated and signed-off by Cardiff before implementation in practice. VIPC will support any download/functionality queries on a practice by practice basis.
- The community consultants, DSN’s and practice teams will review the suite of searches to effectively identify and manage their diabetic patients in the practice on an ongoing basis.
- Patients identified for structured education will be referred into the service as per local Pathway.
- A project manager will be assigned from MSD and the programme will be facilitated by this project lead.
- The community consultants and DSN’s will support clinical education during practice visits and review agreed patients groups at practice level.
- Cardiff and Vale UHB will evaluate project outcomes using National Diabetes Audit data and share this with MSD.
Project Objectives
- Standardise and harmonise ways of working by implementing NICE NG28
- More cost-effective care: Cost effective prescribing, fewer admissions with diabetes complications, reduction in referrals to diabetes services.
- Greater patient access based on need.
- Workforce transformation and stability.
Benefits
Benefit to Patient – Patients feel better supported to manage their condition by having a greater understanding of their condition and the treatment options available to them which supports self-management leading to improved management and a better quality of life.
Benefit to NHS – People delivering diabetes care are able to provide the best support and care possible for people with diabetes by implementing NG28 which will lead to improved patient management and a reduction in the cost burden of managing diabetes. This will support an improved quality of care; a reduction in diabetes related complications and improved self-management.
Benefit to MSD – Supporting Cardiff and Vale University Health Board to improve outcomes for diabetic patients will demonstrate MSD as a trusted partner through deployment of skills and resources to support and facilitate higher quality care for all appropriate patients. The collaboration may benefit MSD indirectly from changes in clinical behaviour as National Diabetes guidelines (NG28) is implemented locally.
Funding
The total project budget is £45,700.00. This is made up from a contribution of £13,200 by MSD and a contribution of £32,500 by Cardiff and Vale University Health Board.
Date of Preparation: January 2022, Extended January 2026 | GB-NON-05449
Read about our range of partnerships through some of our stories:
GB-NON-07473 | Date of Preparation: May 2023



